Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you please solve this questuons for me. i am trying it by myself still need all of the structures and all of the nomenclature.
can you please solve this questuons for me. i am trying it by myself still need all of the structures and all of the nomenclature. 



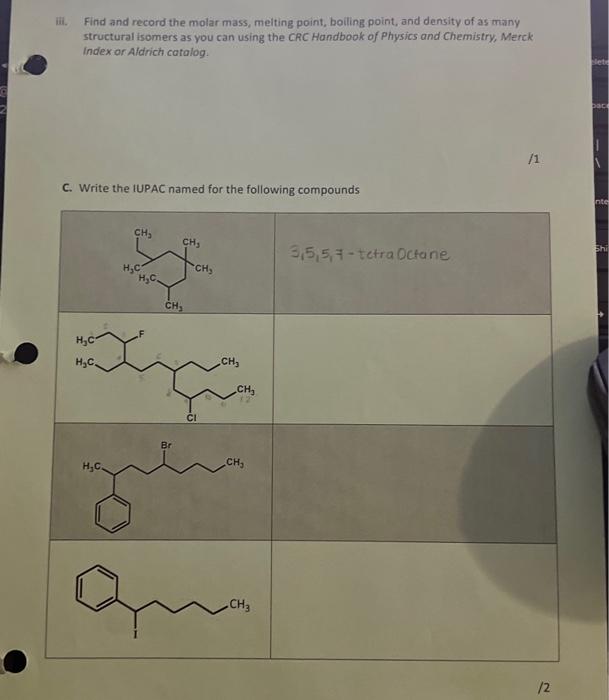
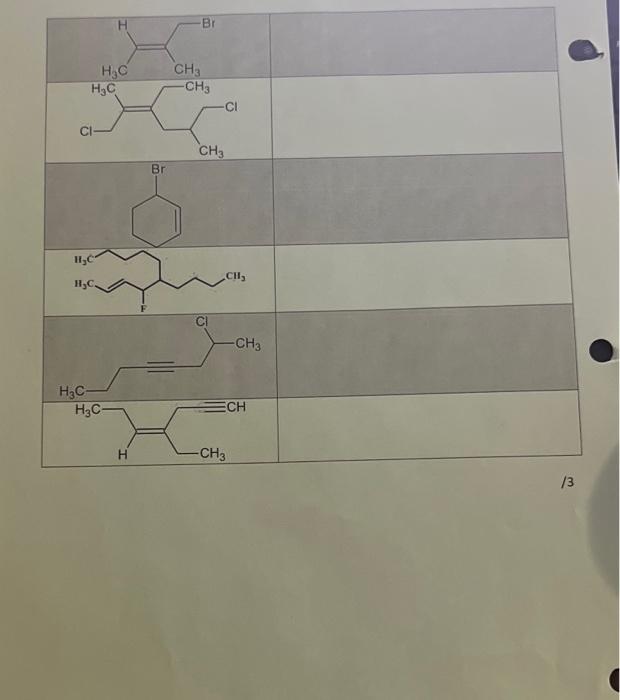
D. Draw the structural formula for the following molecules 6-ethyl-2,2,4-trimethylundecane 4-bromo-3,3-dimethylheptane 4-ethyl-3-nitrononane 2-bromo-2-chloro-1,1,1-trifluorononane 2-chloro-3,3-diethyl-4-phenyl-heptane /2.5 (3E)-3-methylhept-3-ene hepta-1,4-diene (22)-1,6-dichloro-3-ethyl-2,5 dimethylhex-2-ene 6-methylhept-3-yne (7E)-4-methylnon-7-en-1-yne Total /20 A. The molecular formula of pentane and hexane are C3H12, and C6H14 respectively. You will be assigned an alkane by your TA or instructor to work with for this section. Use extra paper if you need it but attach those sheets to the report. i. For your assigned alkane, draw the structural formula for as many isomers as you can below. (Pentane) a. CH3CH2CH2CH2CH3CH3or b. CH3CH2CHCH3CH3 or /2 ii. Name each isomer using IUPAC nomenclature. a. Pentane b. 2-methylbutane c. 2-2-dimethy/pentane.propane iii. Find and record the molar mass, meiting point, boiling point, and density of as many structural isomers as you can using the CRC Handbook of Physics and Chembiry. Merck index or Sigmo-Aldrich catolog. B. The molecular formula of pentene and hexene are C3H10 and C1H2 respectively. You will be assigned an alkene by your TA or instructor to work with for this section. Use extra paper if you need it but attach those sheets to the report. I. For your assigned alkene, draw the structural formula (including the stereochemistry) for as many isomers as you can below (only isomers with a double bond and the correct molecular formula will be correct). a. b. C. ii. Name each isomer using IUPAC nomenclature. iii. Find and record the molar mass, meiting point, boiling point, and density of as many structural isomers as you can using the CRC Handbook of Physics and Chemistry, Merck Index or Aldrich catalog. 1 C. Write the IUPAC named for the following compounds 





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


