Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you plz answer all the questions Thanks A student is asked to design a simple calorimetry experiment to determine the molar enthalpy of combustion
Can you plz answer all the questions 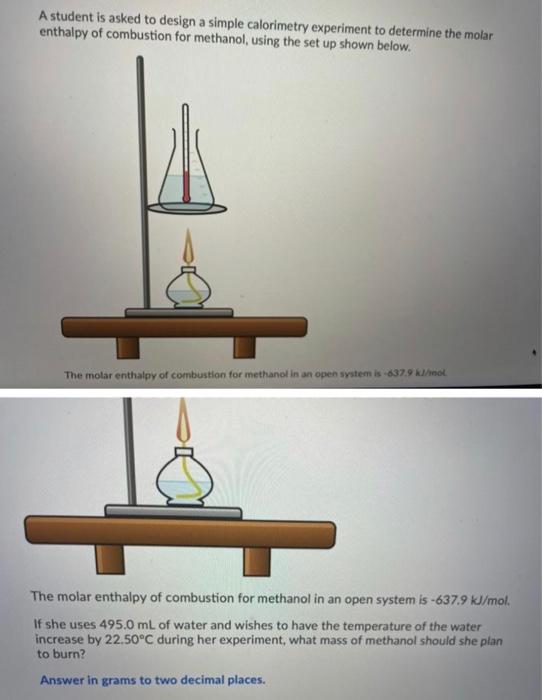
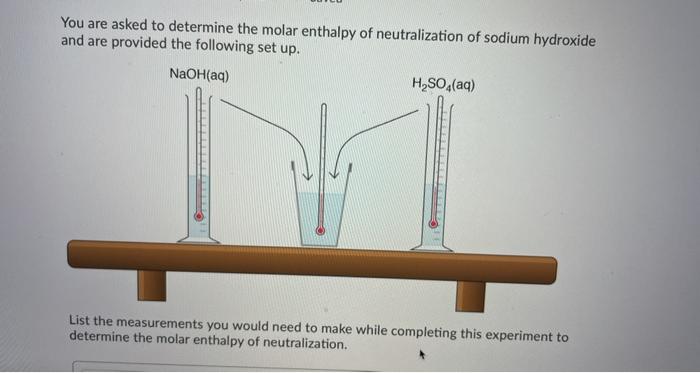
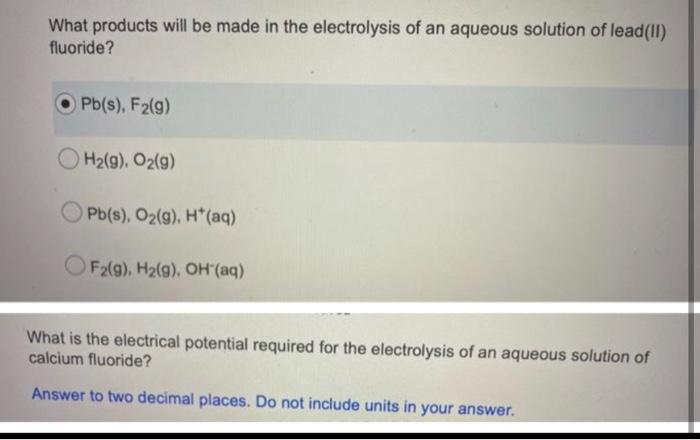
A student is asked to design a simple calorimetry experiment to determine the molar enthalpy of combustion for methanol, using the set up shown below. The molar enthalpy of combustion for methanol in an open system is 637.9k/mol The molar enthalpy of combustion for methanol in an open system is -637.9 kJ/mol. If she uses 495.0 mL of water and wishes to have the temperature of the water increase by 22.50C during her experiment, what mass of methanol should she plan to burn? Answer in grams to two decimal places. You are asked to determine the molar enthalpy of neutralization of sodium hydroxide and are provided the following set up. NaOH(aq) H, SO.(aq) List the measurements you would need to make while completing this experiment to determine the molar enthalpy of neutralization. What products will be made in the electrolysis of an aqueous solution of lead(11) fluoride? Pb(s), F2(g) H2(g). O2(9) Pb(s), O2(g), H(aq) F2(g), H2(g), OH (aq) What is the electrical potential required for the electrolysis of an aqueous solution of calcium fluoride? Answer to two decimal places. Do not include units in your Thanks



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


