Question
Carbon dioxide gas can be decomposed into oxygen gas and carbon 2 points monoxide: 2CO2(g) 02(g) + 2CO(g) AH = 486 kJ. How much
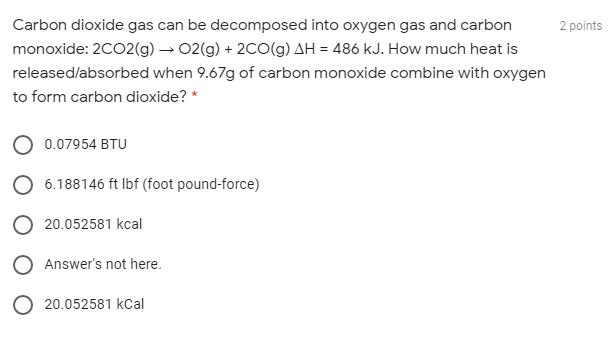
Carbon dioxide gas can be decomposed into oxygen gas and carbon 2 points monoxide: 2CO2(g) 02(g) + 2CO(g) AH = 486 kJ. How much heat is released/absorbed when 9.67g of carbon monoxide combine with oxygen to form carbon dioxide? 0.07954 BTU 6.188146 ft Ibf (foot pound-force) O 20.052581 kcal O Answer's not here. O 20.052581 kCal
Step by Step Solution
3.34 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
967 g of CO combine with Oxygen to form CO2Hence the rea...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Joseph M. Hornback
2nd Edition
9781133384847, 9780199270293, 534389511, 1133384846, 978-0534389512
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



