Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Carbon monoxide is a useful one carbon building block for many industrial processes. Consider the hypothetical reduction of carbon dioxide to carbon monoxide. 2CO(g)
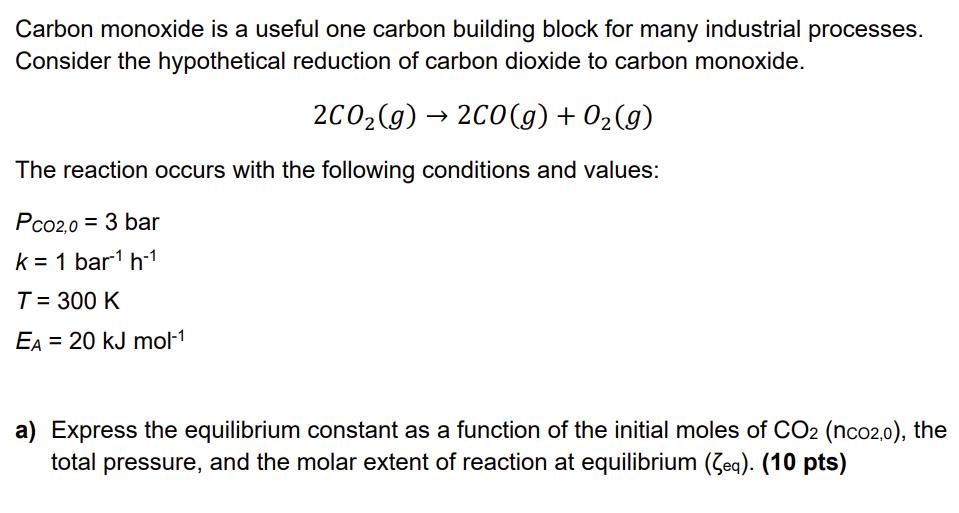

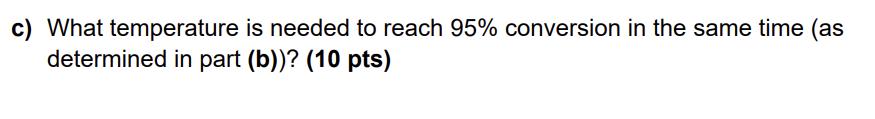
Carbon monoxide is a useful one carbon building block for many industrial processes. Consider the hypothetical reduction of carbon dioxide to carbon monoxide. 2CO(g) 2CO(g) + O(g) The reaction occurs with the following conditions and values: Pc02,0 = 3 bar k = 1 bar1 h-1 T = 300 K EA = 20 kJ mol-1 a) Express the equilibrium constant as a function of the initial moles of CO2 (nco2,0), the total pressure, and the molar extent of reaction at equilibrium (Zeq). (10 pts) b) Determine the time required to reach a total pressure of 4 bar. Assume the written reaction is an elementary step and that the reaction is conducted under constant volume and temperature conditions. (10 pts) c) What temperature is needed to reach 95% conversion in the same time (as determined in part (b))? (10 pts)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


