Answered step by step
Verified Expert Solution
Question
1 Approved Answer
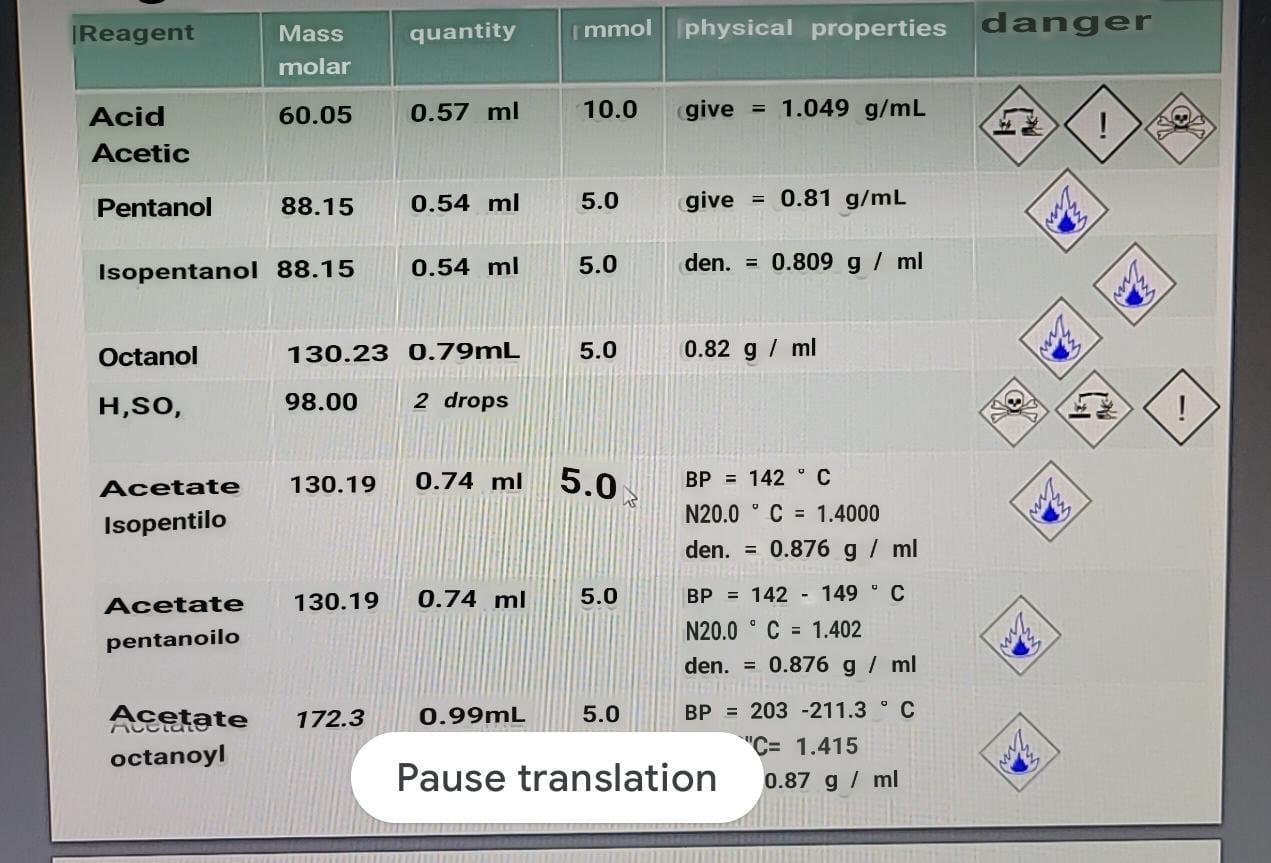
CASE STUDY: SYNTHESIS OF AN ESTER: FISCHER ESTERIFICATION A. Theoretical calculations: Calculate the limiting reactant and the theoretical performance. [Reagent quantity immol physical properties danger
CASE STUDY: SYNTHESIS OF AN ESTER: FISCHER ESTERIFICATION
A. Theoretical calculations: Calculate the limiting reactant and the theoretical performance.
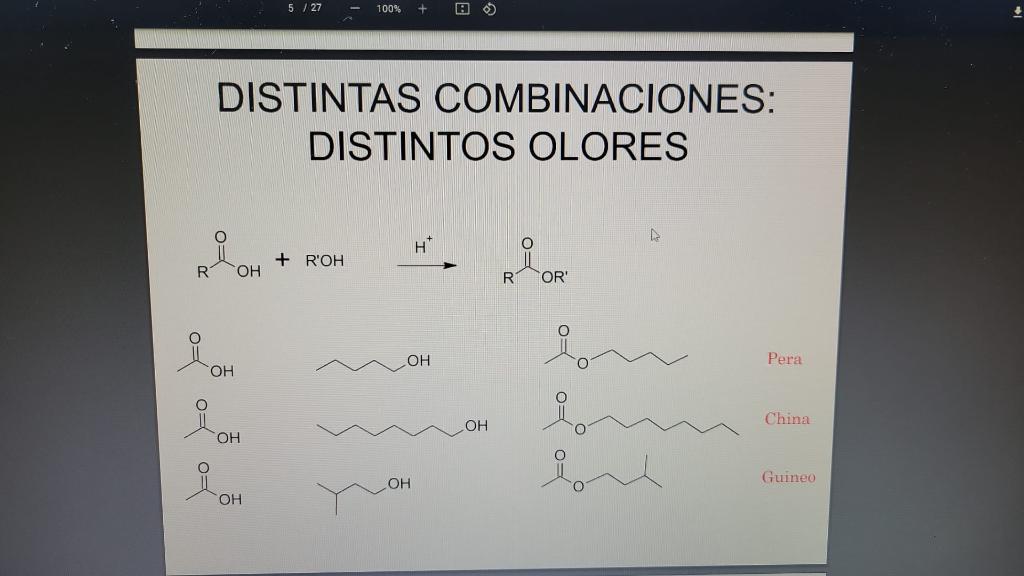
[Reagent quantity immol physical properties danger Mass molar 60.05 0.57 ml 10.0 give = 1.049 g/mL Acid Acetic ! Pentanol 88.15 0.54 ml 5.0 = give 0.81 g/mL Isopentanol 88.15 0.54 ml 5.0 den. = 0.809 g / ml Octanol 130.23 0.79mL 5.0 0.82 g / ml H,SO, 98.00 2 drops ! 130.19 0.74 ml 5.0 Acetate Isopentilo w BP = 142 C N20.0 C = 1.4000 den. = 0.876 g / ml 130.19 0.74 ml 5.0 Acetate pentanoilo BP = 142 - 149C N20.0C = 1.402 den. = 0.876 g / ml W 172.3 0.99mL 5.0 BP = 203-211.3 Acetate octanoyl Pause translation "C= 1.415 0.87 g / ml 5 / 27 100% + DISTINTAS COMBINACIONES: DISTINTOS OLORES H + R'OH R OH R OR Reole Low i OH Pera OH of of op China OH OH ROH OH GuineoStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


