Question
Br 0 N H Assign oxidation numbers to each of the elements in the reaction below. Then compare how the oxidation numbers changed during
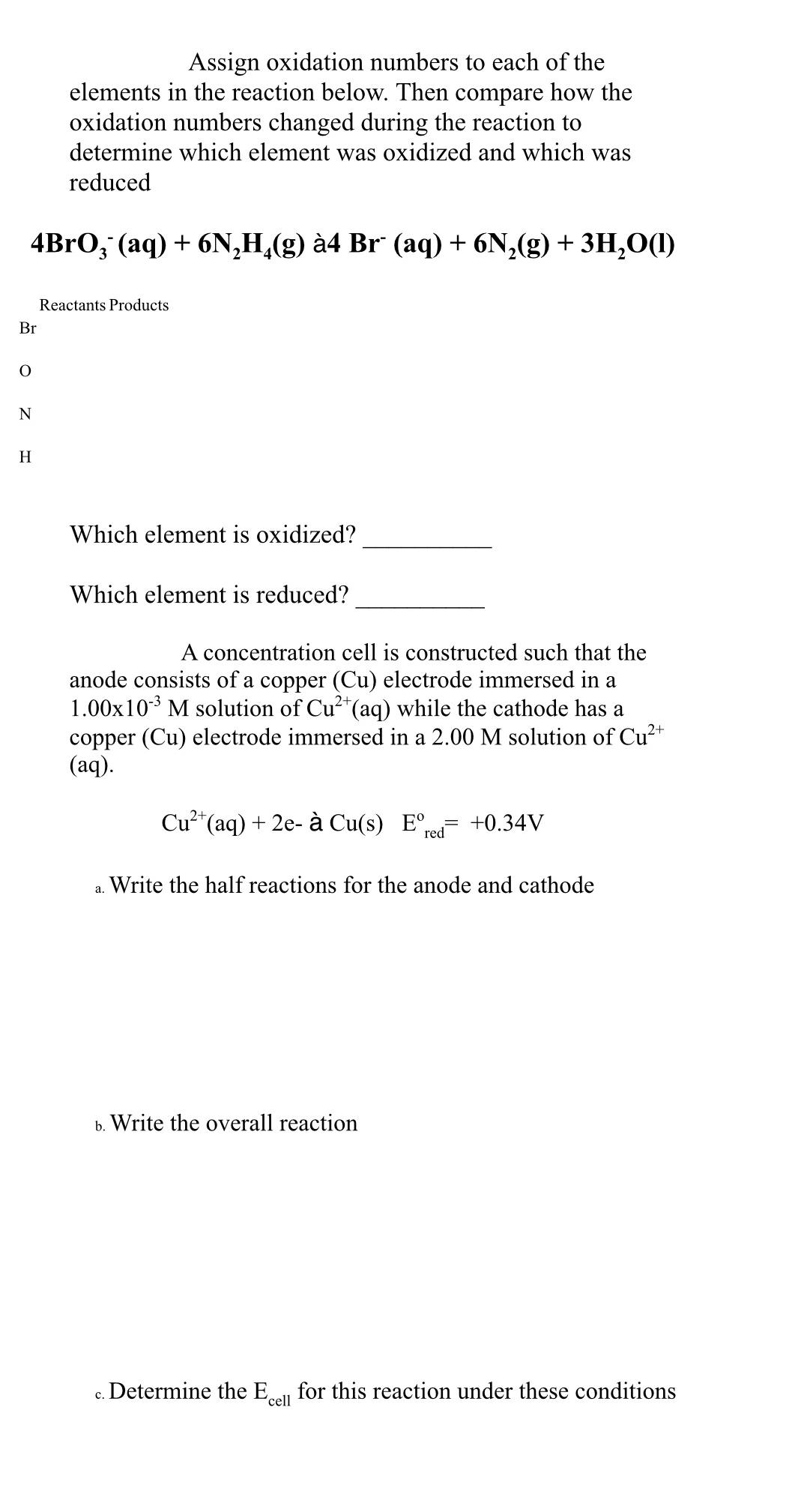
Br 0 N H Assign oxidation numbers to each of the elements in the reaction below. Then compare how the oxidation numbers changed during the reaction to determine which element was oxidized and which was reduced 4BrO3(aq) + 6NH(g) 4 Br (aq) + 6N2(g) + 3H2O(1) Reactants Products Which element is oxidized? Which element is reduced? A concentration cell is constructed such that the anode consists of a copper (Cu) electrode immersed in a 1.00x103 M solution of Cu2+(aq) while the cathode has a copper (Cu) electrode immersed in a 2.00 M solution of Cu2+ (aq). Cu2+ (aq) + 2e- Cu(s) Ered +0.34V a. Write the half reactions for the anode and cathode b. Write the overall reaction c. Determine the E cell for this reaction under these conditions
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Reporting Financial Statement Analysis And Valuation A Strategic Perspective
Authors: James M. Wahlen, Stephen P. Baginski, Mark Bradshaw
8th Edition
1285190904, 978-1305176348, 1305176340, 978-1285190907
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



