Question
Consider the following reaction: Pb(NO3)2(aq) +2 KI (aq) PbI2(s) + 2 KNO3(aq) What volume of a 4.0 M potassium iodide solution is required to
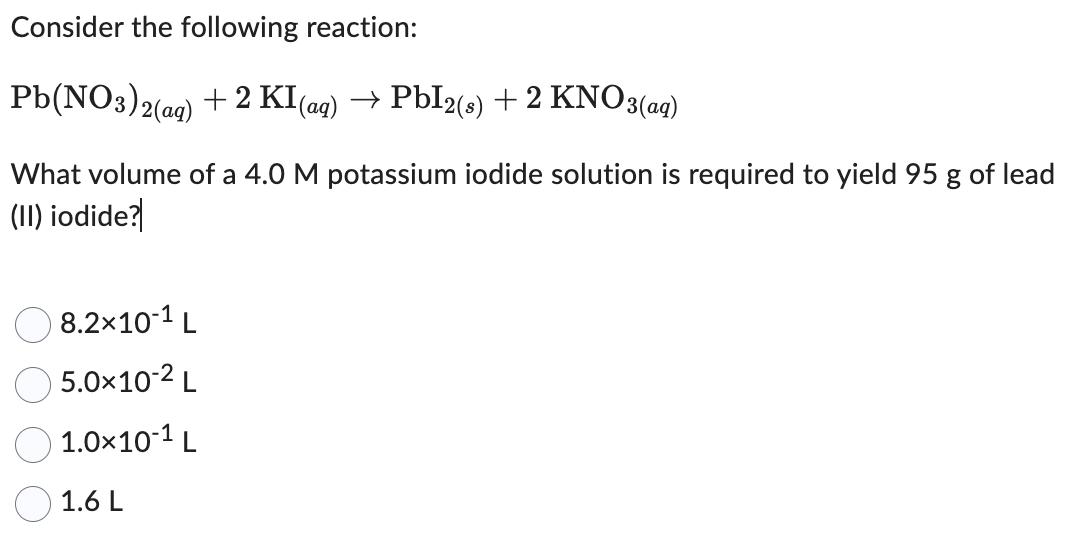
Consider the following reaction: Pb(NO3)2(aq) +2 KI (aq) PbI2(s) + 2 KNO3(aq) What volume of a 4.0 M potassium iodide solution is required to yield 95 g of lead (II) iodide? 8.210-1 L 5.010-2 L 1.010-1 L 1.6 L
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Marketing An Introduction
Authors: Gary Armstrong, Philip Kotler
13th edition
013414953X, 978-0134149530
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



