Answered step by step
Verified Expert Solution
Question
1 Approved Answer
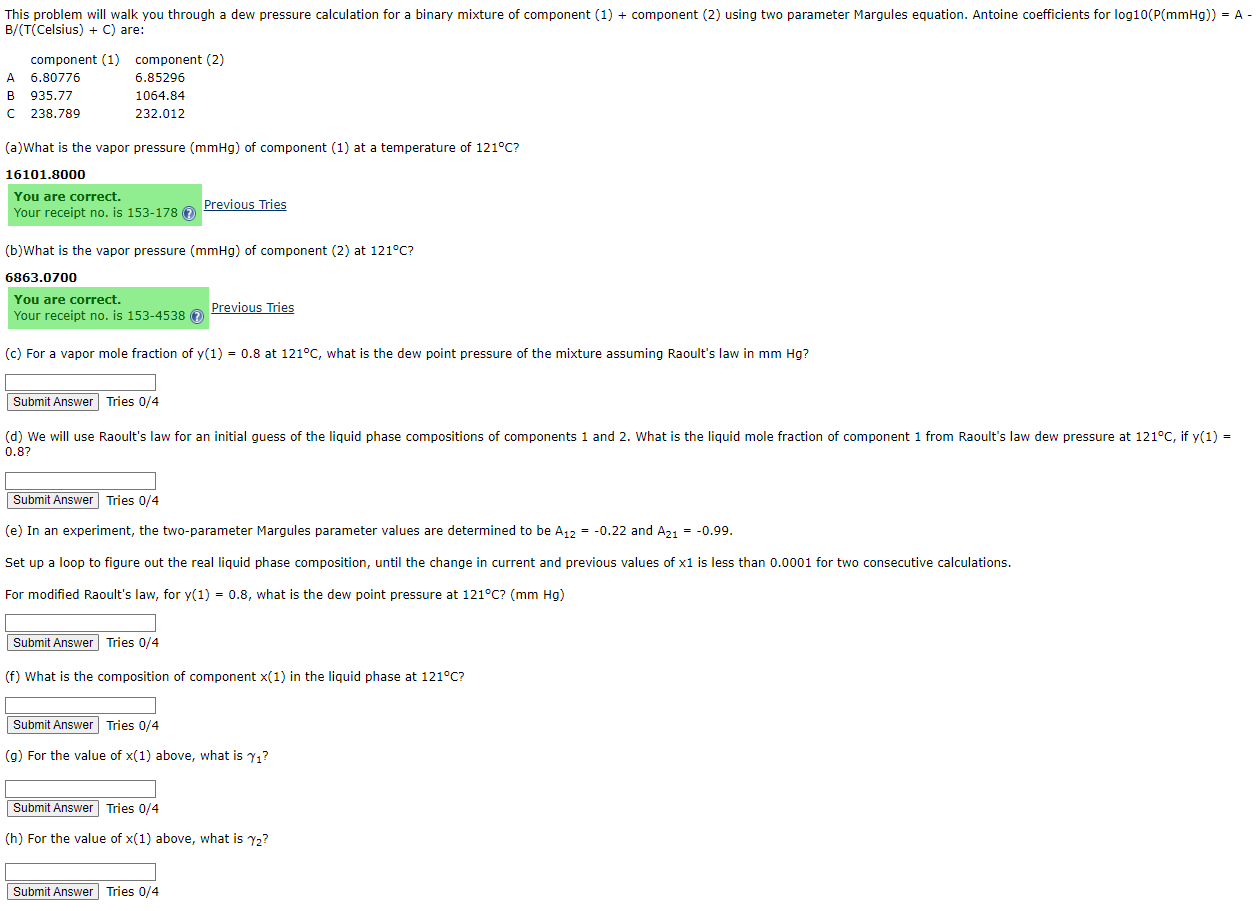
( Celsius ) + C are: ( a ) What is the vapor pressure ) of component ( 1 ) at a temperature of 1
Celsius are:
aWhat is the vapor pressure of component at a temperature of
You are correct.
Your receipt no is Previous Tries
b What is the vapor pressure of component at
You are correct.
Your receipt no is Previous Tries
c For a vapor mole fraction of at what is the dew point pressure of the mixture assuming Raoult's law in
Tries
Submit Answer Tries
e In an experiment, the twoparameter Margules parameter values are determined to be and
Set up a loop to figure out the real liquid phase composition, until the change in current and previous values of is less than for two consecutive calculations.
For modified Raoult's law, for what is the dew point pressure at
Tries
f What is the composition of component in the liquid phase at
Submit Answer Tries
g For the value of above, what is
Tries
h For the value of above, what is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


