Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. a. Acid catalyzed hydration of 2-methylpropene produces only 2-methyl-2- propanol. However, acid catalyzed of 1,2-dimethylcyclohexene produces two products, ie, a mixture of stereoisomers.

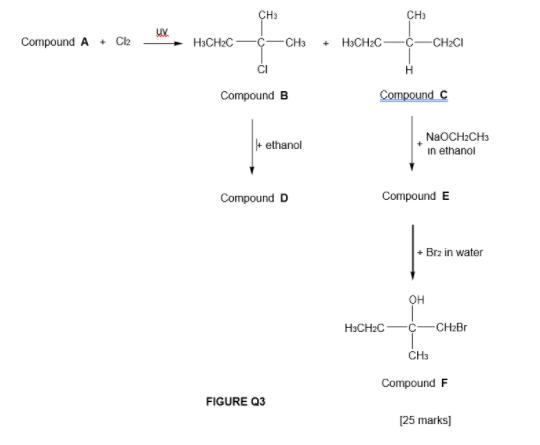
3. a. Acid catalyzed hydration of 2-methylpropene produces only 2-methyl-2- propanol. However, acid catalyzed of 1,2-dimethylcyclohexene produces two products, ie, a mixture of stereoisomers. Using your knowledge in organic chemistry, discuss the regioselective and stereoselective nature of the acid catalyzed hydration based on reaction mechanism. [15 marks) b Compound A reacts with Clz in the presence of ultraviolet (UV) light to produce compounds B and C as shown in FIGURE Q3. Compound B reacts with ethanol at 60C, to fom compound D (major product). Meanwhile compound C reacts with NaOCH,CH, in ethanol to form compound E. Compound E produces compound F when reacts with Br, in water. Using your knowledge in b. organic chemistry, explain in details the following. The constitutional isomers of compounds B and C, if any. The chirality of compounds B, C, D, E and F. If there islare enantiomer(s) among these compounds, show details of working steps in giving IUPAC name to the enantiomer(s). The difference in boiling point between compounds A, C and F. CH: CH) Compound A + C2 H3CH2C- CH3 + HCH:C- CH2CI CI Compound B Compound C NaOCH:CH3 + ethanol in ethanol Compound D Compound E + Brz in water H>CH2C- CH2Br CH Compound F FIGURE Q3 [25 marks]
Step by Step Solution
★★★★★
3.36 Rating (171 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


