Question
Consider the following side chains CH3 CH3 glycine leucine H. phenylalanine tryptophan 2: NH ysine pK, 11.0 glutamic acid pk, 4.1 aspartic acid pK, 4.1
Consider the following side chains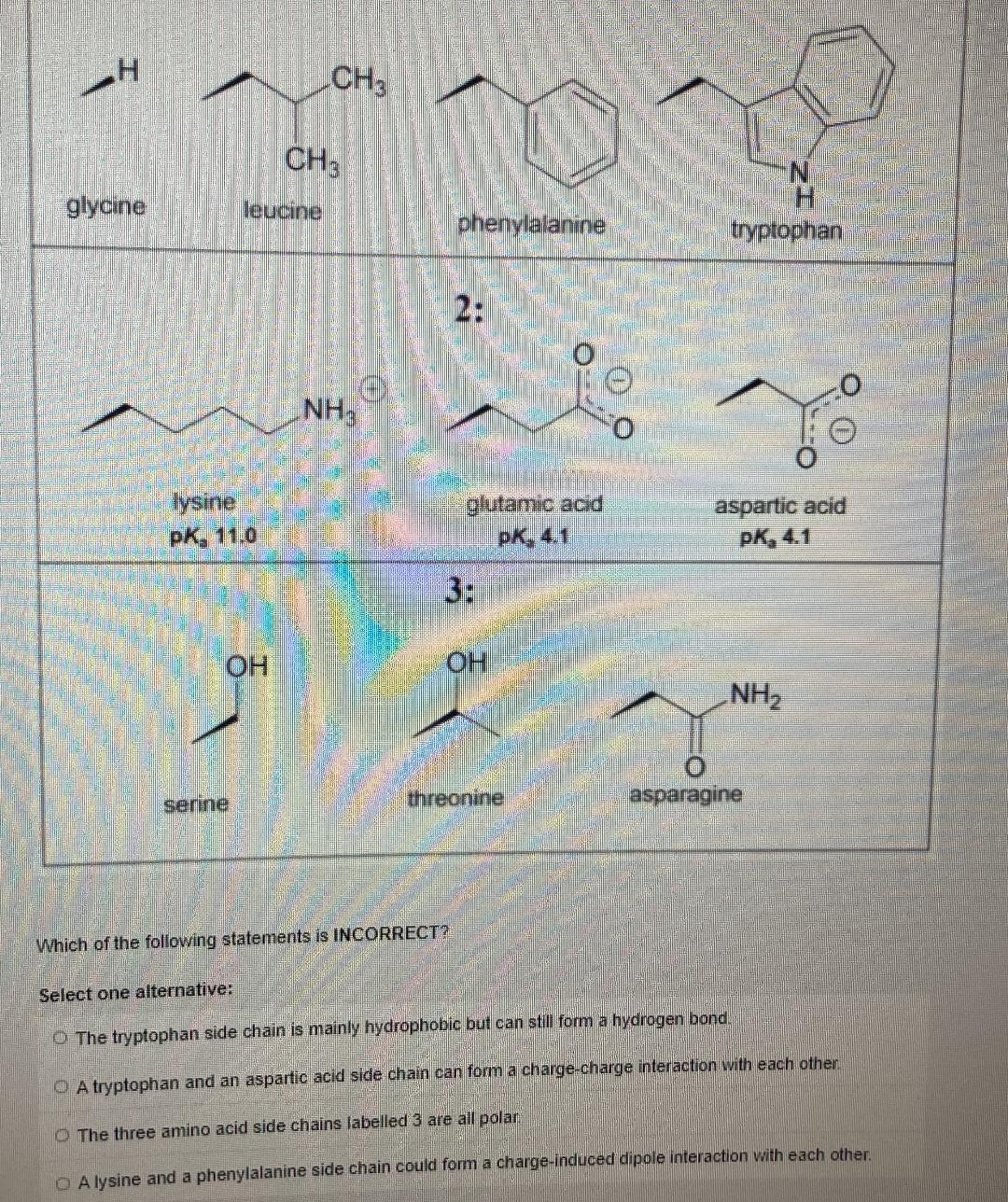
CH3 CH3 glycine leucine H. phenylalanine tryptophan 2: NH ysine pK, 11.0 glutamic acid pk, 4.1 aspartic acid pK, 4.1 3: HO OH NH2 serine, threonine asparagine Which of the following statements is INCORRECT? Select one alternative: O The tryptophan side chain is mainly hydrophobic but can still form a hydrogen bond. O A tryptophan and an aspartic acid side chain can form a charge-charge interaction with each other. O The three amino acid side chains labelled 3 are all polan O A lysine and a phenylalanine side chain could form a charge-induced dipole interaction with each other ()
Step by Step Solution
3.44 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
The Hbond is an electrostatic interaction between hydrogen atom of a molecular fragment XH of a mole...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Microbiology A Systems Approach
Authors: Marjorie Kelly Cowan
4th edition
978-007340243, 73402435, 978-0073402437
Students also viewed these Biology questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



