Answered step by step
Verified Expert Solution
Question
1 Approved Answer
CH3OH(l)CH4(g)+1/2O2(g). Part A Calculate rH298. Use required data from the Tables available under Module 0: Important Files in Canvas. Express your answer using two decimal
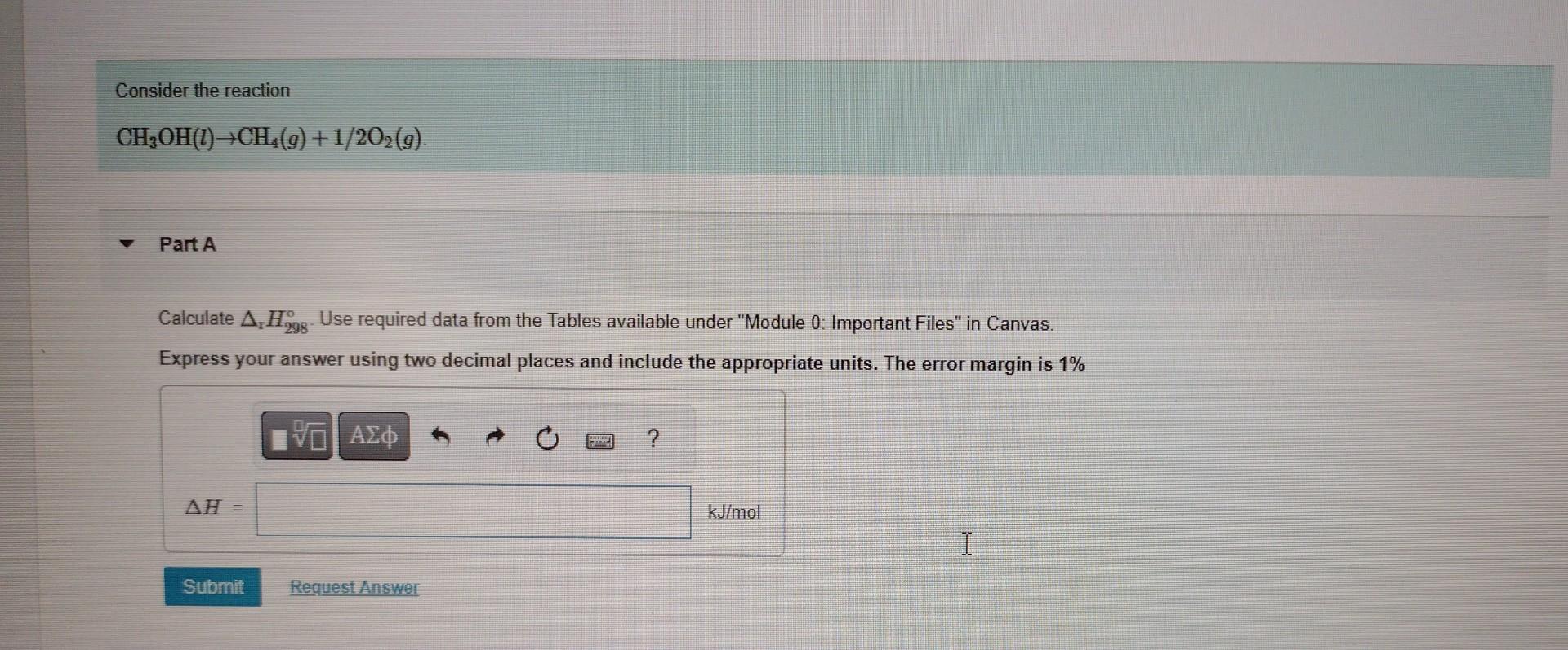
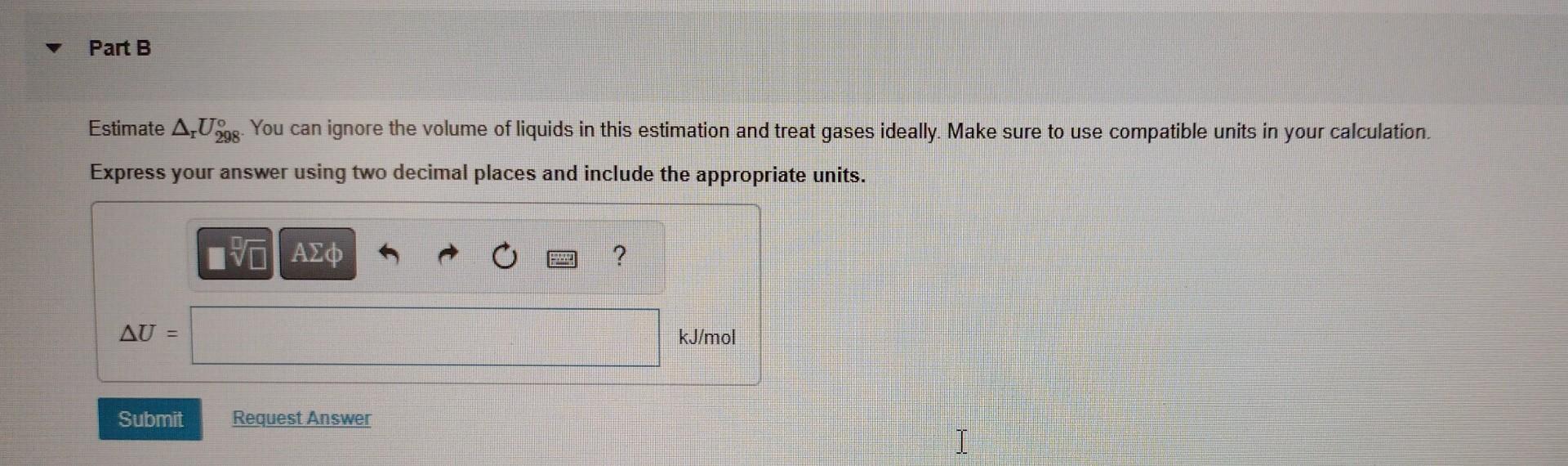

CH3OH(l)CH4(g)+1/2O2(g). Part A Calculate rH298. Use required data from the Tables available under "Module 0: Important Files" in Canvas. Express your answer using two decimal places and include the appropriate units. The error margin is 1% Estimate rU298 You can ignore the volume of liquids in this estimation and treat gases ideally. Make sure to use compatible units in your calculation. Express your answer using two decimal places and include the appropriate units. Choose the equation that would allow you to obtain H at 500C and 1 bar. H298298773(Cp,m(CH4)+1/2Cp,m(O2)Cp,m(CH3OH))dTH298298773(Cp,m(CH4)1/2Cp,m(O2)+Cp,m(CH3OH))dTH298+298773(Cp,m(CH4)+1/2Cp,m(O2)Cp,m(CH3OH))dTH298+298773(Cp,m(CH4)1/2Cp,m(O2)+Cp,m(CH3OH))dT
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


