Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Change for the dissolution of ammonium chloride J/K.mol 6. The average Gibb's Energy change for the dissolution of ammonium chloride Table 2 - Thermodynamic data
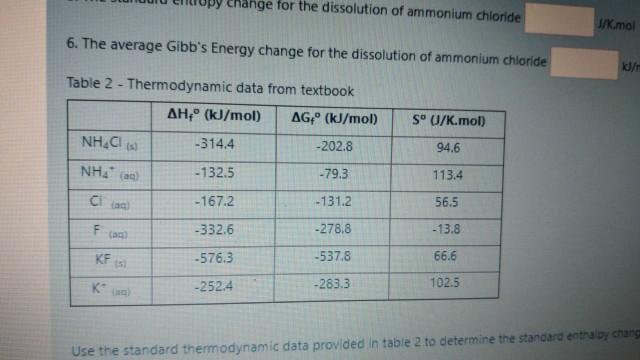
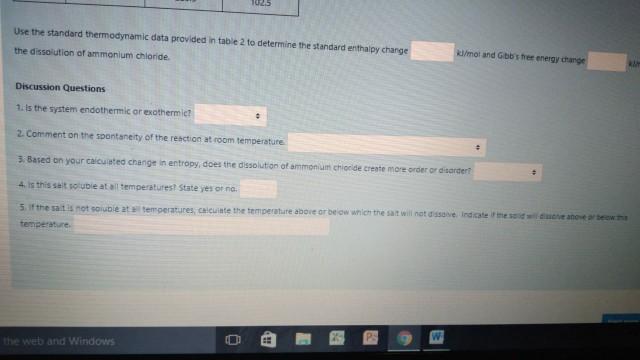
Change for the dissolution of ammonium chloride J/K.mol 6. The average Gibb's Energy change for the dissolution of ammonium chloride Table 2 - Thermodynamic data from textbook AH" (kJ/mol) AG(kJ/mol) S 01/K.mol) NHACI -314.4 -202.8 94.6 NHA - 132.5 -79.3 113.4 - 167.2 -131.2 56.5 F -332.6 -278.8 - 13.8 KF -576.3 -537.8 66.6 -252.4 -283.3 102.5 Use the standard thermodynamic data provided in table 2 to determine the standard entra by chans 1025 Use the standard thermodynamic data provided in table 2 to determine the standard enthalpy change the dissolution of ammonium chloride mot and Goose energy change Discussion Questions 1. Is the system endothermic or exothermiet 2. Comment on the spontaneity of the reaction at room temperature 3. Based on your cated change in entropy, does the dissolution of ammonium chloride create more order or darder 4. Is this set ouble at all temperatures! State yes or na 5. If the saitis not souble at temperatures, calculate the temperature above or below which the sat will not done Indicates than a beth temperature PS w the web and Windows
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


