Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Characteristic X-rays and Auger Electrons Characteristic X-rays are emitted when outer-shell electrons fill a vacancy in the inner shell of an atom, releasing X-rays

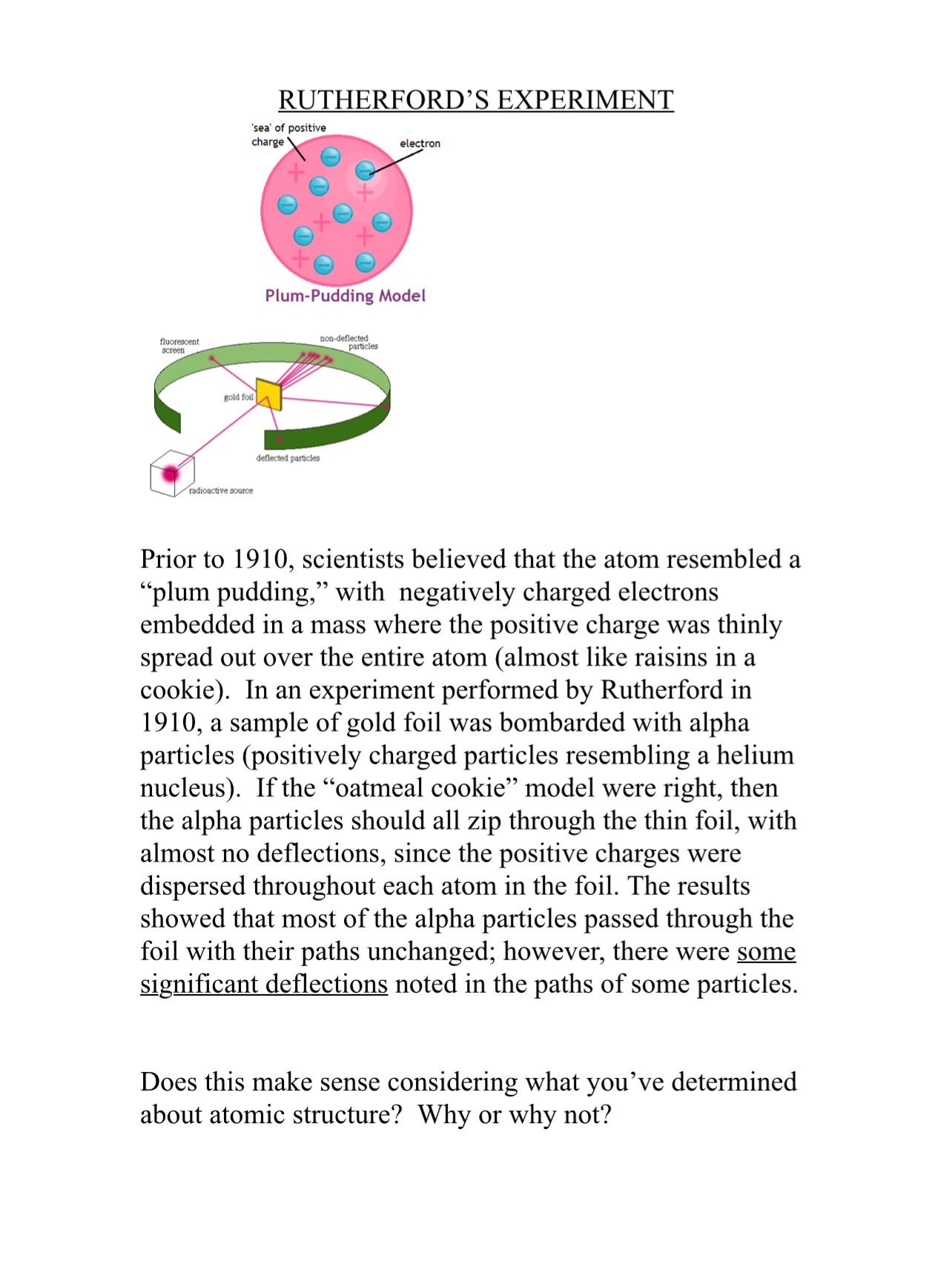
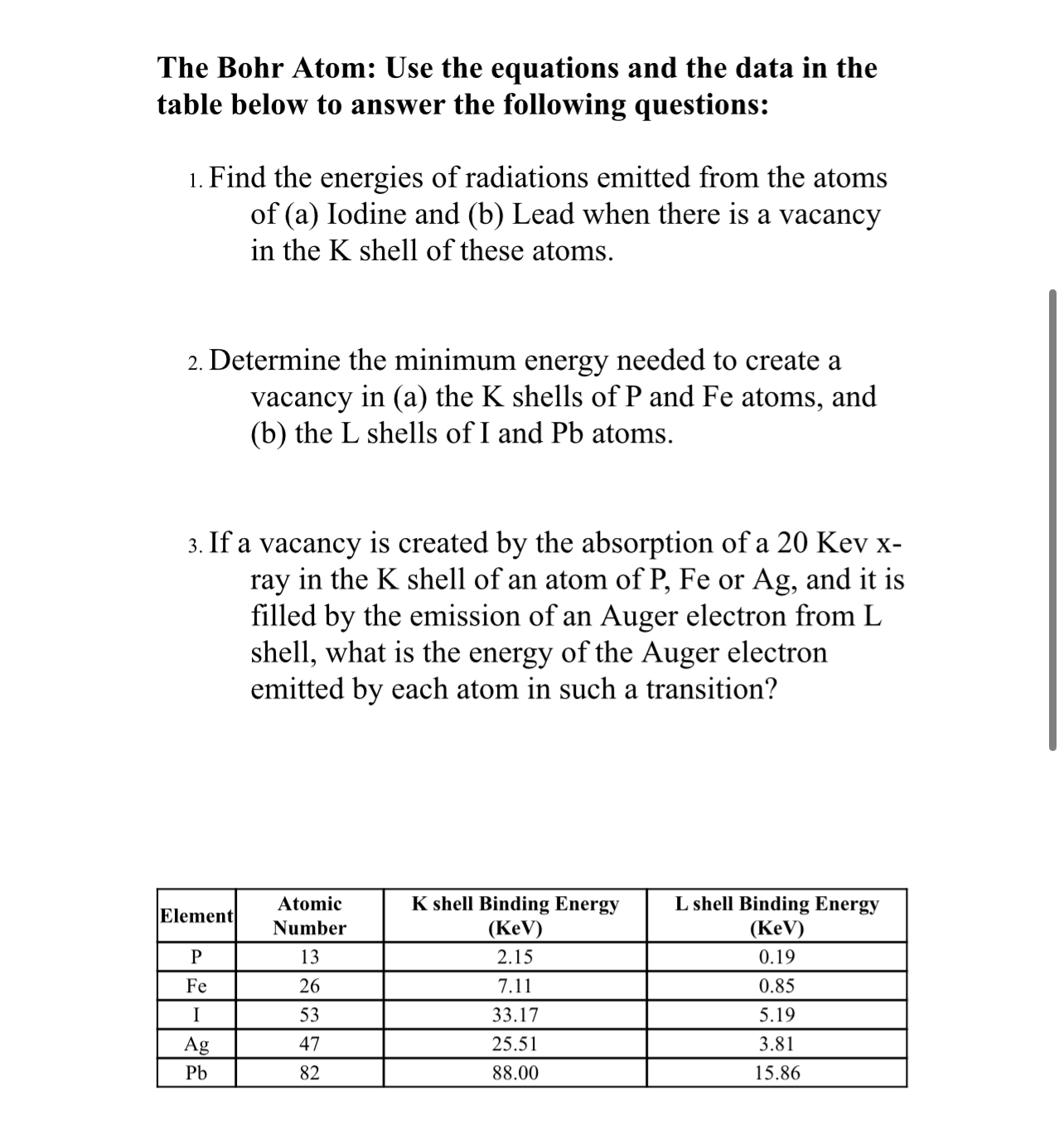
Characteristic X-rays and Auger Electrons "Characteristic X-rays are emitted when outer-shell electrons fill a vacancy in the inner shell of an atom, releasing X-rays in a pattern that is "characteristic" to each element." (Just as the energy associated with each electron shell of an atom is unique to that atom.) The energy of the characteristic x- ray is calculated as the difference between the binding energies of the 2 electron shells. = AE E EK-EL An alternative to characteristic x-ray production is Auger election emission. When an inner shell vacancy is created, an outer shell electron drops in to fill the vacancy. The energy difference between the 2 shells ("excitation energy") is transferred to an outer electron shell, resulting in the release of a second electron (the Auger electron) from the atom. The energy of the Auger electron is calculated as: = AE E-E2-E3 (where E,... E = energy Mass-Energy Equivalence (E=mc) Mass can bed "concentrated energy." If you were to perform the calculations, you would find that a 20 g marble is equivalent to a 500 kiloton hydrogen bomb! The difficulty lies in having the ability to convert the mass to energy. In order to release this energy, a matter-antimatter "annihilation" event needs to occur. Unfortunately, there is not enough anti-matter to make this occur on a routine basis. If one electron were to interact with its anti-matter "partner" (actually, a positron), the mass of the positron and electron could be converted to energy using the E=mc equation. (A) Using the mass of an electron (9.11 x 10-31 kg), and c = 3x108 m/sec (and considering there are an electron AND a positron), calculate the number of joules (J) of energy this would represent. (B) If 1J = 6.24 x 1018 eV, and 106 eV = 1 MeV, calculate the amount of energy (in MeV) produced by the annihilation event associated with the positron-electron interaction. fluorescent screen gold foil radioactive source RUTHERFORD'S EXPERIMENT 'sea' of positive charge electron Plum-Pudding Model deflected particles non-deflected particles Prior to 1910, scientists believed that the atom resembled a "plum pudding," with negatively charged electrons embedded in a mass where the positive charge was thinly spread out over the entire atom (almost like raisins in a cookie). In an experiment performed by Rutherford in 1910, a sample of gold foil was bombarded with alpha particles (positively charged particles resembling a helium nucleus). If the oatmeal cookie model were right, then the alpha particles should all zip through the thin foil, with almost no deflections, since the positive charges were dispersed throughout each atom in the foil. The results showed that most of the alpha particles passed through the foil with their paths unchanged; however, there were some significant deflections noted in the paths of some particles. Does this make sense considering what you've determined about atomic structure? Why or why not? The Bohr Atom: Use the equations and the data in the table below to answer the following questions: 1. Find the energies of radiations emitted from the atoms of (a) Iodine and (b) Lead when there is a vacancy in the K shell of these atoms. 2. Determine the minimum energy needed to create a vacancy in (a) the K shells of P and Fe atoms, and (b) the L shells of I and Pb atoms. 3. If a vacancy is created by the absorption of a 20 Kev x- ray in the K shell of an atom of P, Fe or Ag, and it is filled by the emission of an Auger electron from L shell, what is the energy of the Auger electron emitted by each atom in such a transition? Element Atomic Number K shell Binding Energy (KeV) L shell Binding Energy (KeV) P 13 2.15 0.19 Fe 26 7.11 0.85 I 53 33.17 5.19 Ag 47 25.51 3.81 Pb 82 88.00 15.86
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


