Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Characteristics of Selected Elements Element Symbol Number Melting Point(C) Aluminum Al 13 26.98 2.71 FCC 0.143 0.053 3+ 660.4 Argan Ar 18 39.95 Inert
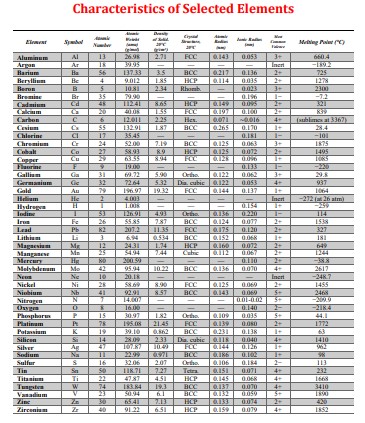

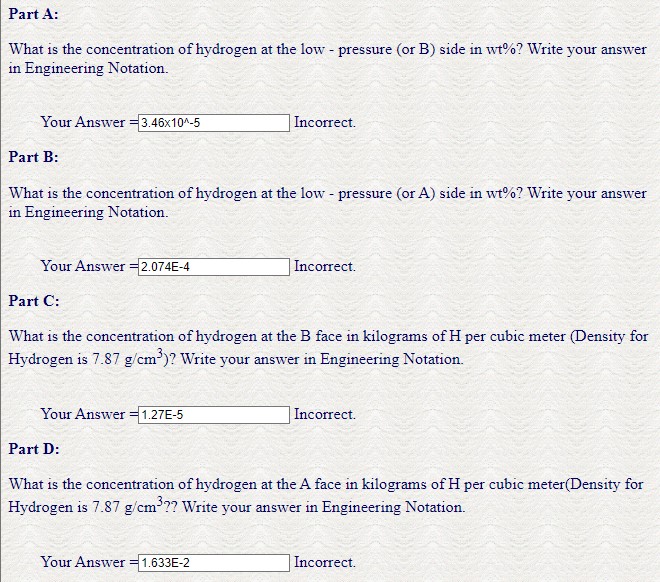


Characteristics of Selected Elements Element Symbol Number Melting Point("C) Aluminum Al 13 26.98 2.71 FCC 0.143 0.053 3+ 660.4 Argan Ar 18 39.95 Inert -189.2 Barium Ba 56 137.33 3.5 BCC 0.217 0.136 2+ 725 Beryllium Be 4 9.012 185 HCP 0114 0.035 2+ 1278 Berun B 5 10.81 2.34 Rhomb 0.023 3+ 2300 Bromine Br 35 79.90 - 0.196 1- -7.2 Cadmium Cd 48 112.41 8.65 HCP 0.149 0095 2+ 321 20 40.08 1.55 FCC 6.197 0.100 2+ K39 Carbon C 6 12.011 2.25 Hes 0.071 -0.016 4+ (sublimes at 3367) Cesium Cx 55 132.91 1.87 BCC 0.265 0.170 + 28.4 Chlorine 17 35.45 0.181 1- 101- Chromium C 24 52.00 7.19 BCC 0.125 0.063 3+ 1875 Cobalt Co 27 58.93 8.9 HCP 0.125 0.072 2+ 1495 Copper Cu 29 63.35 8.94 FCC 0.128 0.096 1+ 1085 Flaurine F 9 19.00 0.133 -2930 Galliam Gia 31 69.72 5.90 Ortho 0.122 0.062 3+ 29.8 Germanium Ce 32 72.64 5.32 Dia. cubic 0.122 0.053 4+ 937 Gold 79 196.97 19.32 FCC 0.144 0.137 1064 He 2 4.003 - Incr -272 (26 am) Hydrogen H 53 126.91 - 4.93 1+ -259 Ortho 0.136 0.220 T- 114 Fe 26 55.85 7.87 BCC 0.124 0.077 2+ 1538 Lead Pb 82 207.2 11.35 FCC 0.175 0.120 2+ 327 Lithium 3 6.94 0.534 BCC 0.152 0.068 181 Magnesium Mr 12 24.31 1.74 HCP 0.160 0.072 2+ 649 Manganese Ma 25 54.94 2.44 Cubic 6.112 0.067 2+ 1244 Mercury He 80 200.59 0.110 2+ -38.8 Molybdenum Mo 42 95.94 10.22 BCC 0.136 0.070 4+ 2617 Ne 10 20.18 Inert -248.7 Nickel Ni 28 58.69 8.90 FCC 0.125 0.069 2+ 1455 Niobium Nb 41 92.91 8.57 BCC 0.143 0.069 5+ 2468 Nitrogen N T 14.007 - - 0.01-0.02 5+ -2009 Oxygen 0 16.00 - 0.140 2- -2184 Phosphorus P 15 30.97 182 Ortho 0.109 0.035 5+ 44.1 Platinam P 78 195.08 21.45 FCC 0.139 0.060 2+ 1772 Potassium K 19 39.10 0.862 BCC 0.231 0.138 1+ 63 Silicon Si 14 28.09 2.33 Dia cubic 0.118 0.040 4+ 1410 Silver A 47 107.87 10.49 FCC 0.144 0.126 1+ 962 Sodium Na 11 22.99 0971 BCC 0.186 0.102 1+ 98 Sulfur S 16 32.06 207 Ortho. 0.106 0.184 2- 113 Tin Sa 50 118.71 7.27 Tetra 0.151 0.071 4+ 232 Titanium Ti 22 47.87 451 HCP 0.145 0.068 Tungsten W 34 183.84 19.3 BCC 0.137 0.070 4+ 4+ 1668 3410 Vanadium V 23 50.94 6T BCC 0.132 0.059 5+ 1890 30 65.41 7.13 HCP 0.133 0.074 2+ 420 Zirconium 40 91.22 6.51 HCP 0.159 0.079 4+ 1852 Homework Problem HW5_2 Attemps Used = 2/8 Due Date Sun, Feb 12, 2023, 23:59:00 Current Time Fri, Feb 10, 2023, 16:58:50 Problem: When alpha-iron is subjected to an atmosphere of hydrogen gas, the concentration of hydrogen in the iron, CH (in weight percent), is a function of hydrogen pressure, PH2 (in Mpa), and asbsolute temperature (T) according to CH= 1.34 X 102 sqrt(PH2) exp(- (27.2KJ/mol)/RT) Consider a thin iron membrane with the thickness and temperature listed below. Calculate the flux in (kg/(m-s)) through this membrane if the hydrogen pressure on one side of the membrane is 0.17 MPa, and on the other side it is 6.1MPa, utilizing the densities provided by your examination booklet. Assume the values for Do is 4.9x107 m/s and Qd is 13.4KJ/mol. --Given Values-- Membrane Thickness (mm) 3.5 Temperature in Celsius = 302 Part A: What is the concentration of hydrogen at the low-pressure (or B) side in wt%? Write your answer in Engineering Notation. Your Answer =3.46x10^-5 Part B: Incorrect. What is the concentration of hydrogen at the low-pressure (or A) side in wt%? Write your answer in Engineering Notation. Your Answer =2.074E-4 Part C: Incorrect. What is the concentration of hydrogen at the B face in kilograms of H per cubic meter (Density for Hydrogen is 7.87 g/cm)? Write your answer in Engineering Notation. Your Answer =1.27E-5 Part D: Incorrect. What is the concentration of hydrogen at the A face in kilograms of H per cubic meter(Density for Hydrogen is 7.87 g/cm?? Write your answer in Engineering Notation. Your Answer 1.633E-2 = Incorrect. Part E: What is the concentration gradient (C/X) in kg/m? Write your answer as positive to the thousandth place. Your Answer -2.387 Part F: = Incorrect. Calculate the diffusion coefficient, D, in m/s at the specified temperature. Write your answer in Engineering Notation. Your Answer 4.02E-8 Part G: Incorrect. Compute the diffusion flux in kg/m-s. Write your answer in Engineering Notation. Your Answer 9.61E-8 Incorrect. Your Score=0/100 A Tabulation of Diffusion Data Diffasing Species Host Metal D) Activation Energy Q/kJ/mol) Calculated Value TC) D(x) Interstitial Diffusion a-Fe (BCC) 1.1 10 87.4 500 1.4 10-12 900 1.4 10" Y-Fe (FCC) 2.3 10 148 900 5.9 10-12 1100 5.3 x 10-1 N a-Fe (BCC) 5.0 10 77 500 3.1 10-12 Y-Fe (FCCY 9.110 168 500 4.010 Self-Diffusion Fe a-Fe (BCC) 2.8 10 251 500 3.0 10-1 900 1.810-1 Fe Y-Fe (FCCY 5.0 10-5 284 900 1.110-17 1100 7.7 10- Cu Cu 2.5 107 200 500 7510 Al 2.310 144 500 Mg- Mg 1.5x10 136 500 Zn- Zn 1.5105 94 500 Mo 1.810 461 500 4.2 10-14 9.610 6.6 10-12 1.210 NI Ni 1.9x10 285 500 1.0 1033 Interdiffusion (Vacancy) Zn- Cu 2.4 10 189 500 4.0x108 Cu Zn 2.1x10 124 500 8.7 x 10 Cu Al 6.510 136 500 4.2 10 Mg- Al 1.2x10 Cu Ni 2.710 256 Ni Cu 1.9 10 1.9x10 1.310-22 5.4 10- Tabulation of Error Function Values 130 500 500 230 500 erf(z) erf(z) erf(z) 0 0.55 0.5633 0.9340 0.025 0.0282 0.60 0.6039 1.4 0.9523 0.05 0.0564 0.65 0.6420 1.5 0.9661 0.10 0.1125 0.70 0.6778 1.6 0.9763 0.15 0.1680 0.75 0.7112 1.7 0.9838 0.20 0.2227 0.80 0.7421 1.8 0.9891 0.25 0.2763 0.85 0.7707 1.9 0.9928 0.30 0.3286 0.90 0.7970 2.0 0.9953 0.35 0.3794 0.95 0.8209 2.2 0.9981 0.40 0.4284 1.0 0.8427 2.4 0.9993 0.45 0.4755 1.1 0.8802 2.6 0.9998 0.50 0.5205 12 0.9103 2.8 0.9999 Adario from Caller & Baby
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


