Answered step by step
Verified Expert Solution
Question
1 Approved Answer
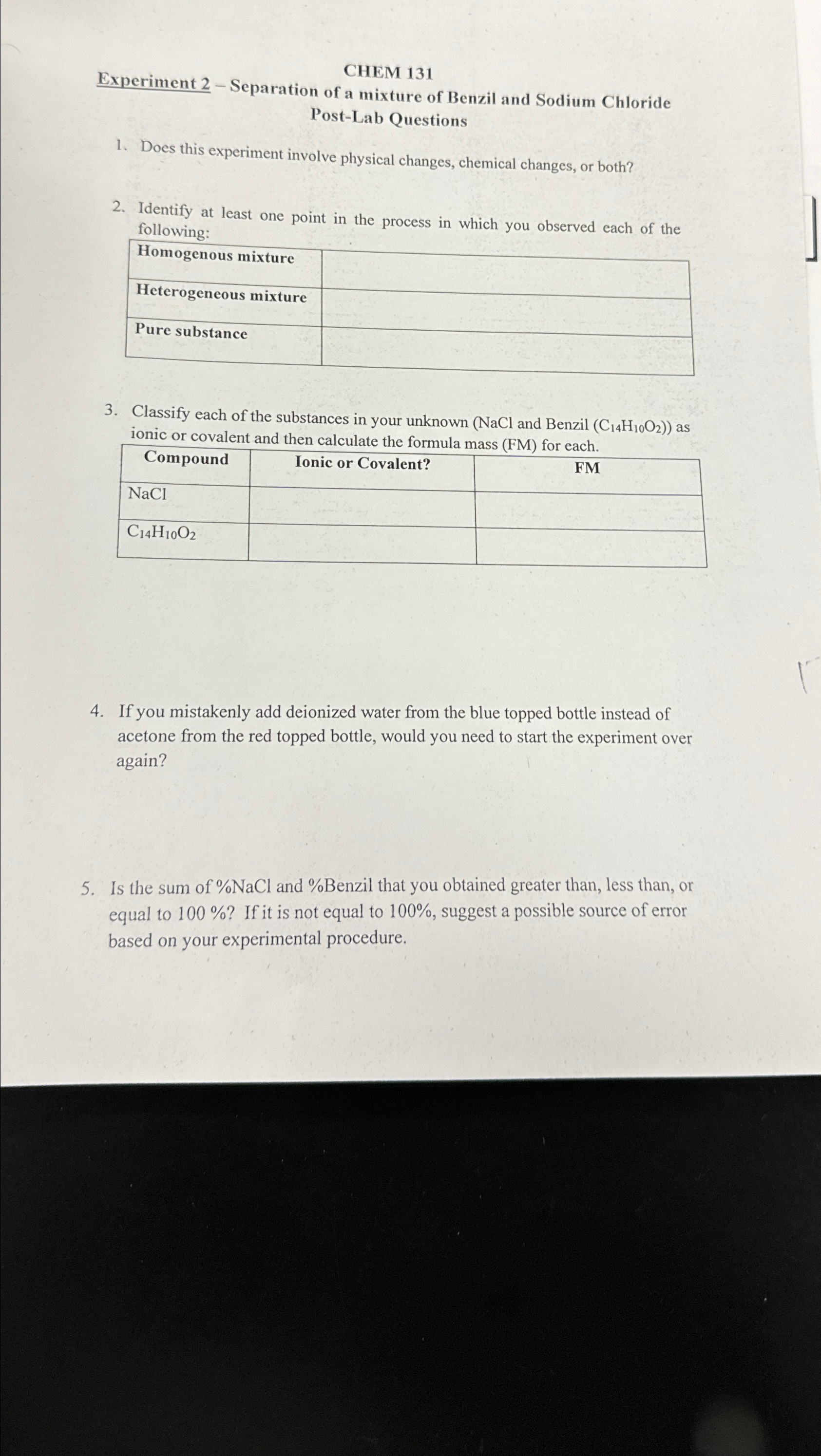
CHEM 1 3 1 Experiment 2 - Separation of a mixture of Benzil and Sodium Chloride Post - Lab Questions Does this experiment involve physical
CHEM
Experiment Separation of a mixture of Benzil and Sodium Chloride PostLab Questions
Does this experiment involve physical changes, chemical changes, or both?
Identify at least one point in the process in which you observed each of the following:
tableHomogenous mixture,Heterogeneous mixture,Pure substance,
Classify each of the substances in your unknown and Benzil : as ionic or covalent and then calculate the formula mass FM for each.
tableCompoundIonic or Covalent?,FM
If you mistakenly add deionized water from the blue topped bottle instead of acetone from the red topped bottle, would you need to start the experiment over again?
Is the sum of NaCl and Benzil that you obtained greater than, less than, or equal to If it is not equal to suggest a possible source of error based on your experimental procedure.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


