

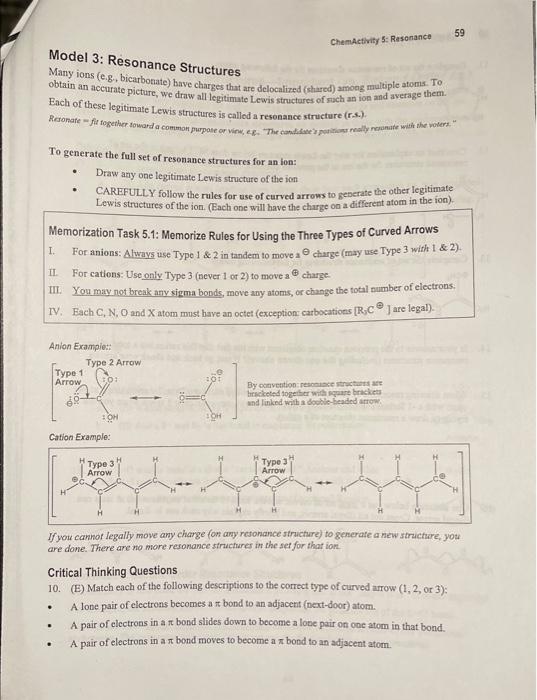
ChemActivity 5: Resonance PART A: DRAWING RESONANCE STRUCTURES (Wby is baking soda [NaHCO] ] w wak base even though it bas an O with a -1 formal eharge7) Model 1: +1 Counterions We have encouatered many - 1 anions in this conne: HO,Cl,(CH2hN,HCO2,C, ete. If you go to the stockroom you will not find a botile of " HO - (bydroxide) on the shelf becaise every anion must exist in close proximity to a counterion, an ion that balances out the charge In this book we will generally assume that positive counterions are inert and that their only function is to balance the negative charge of the anion. Also assume that all ions appearing in this book have a counterion, even if ope is not specified. Critical Thinking Questions 1. Which elements on the periodic table (other than Hi) are likely to form a +1 cation? Li, Na, K, Rb, CssFr 2. You will not find "bydroxide" in the stockroom, but you will find sodium hydroxide (NaOH) and potassium hydroxide (KOH). Lithium hydroxide (LiOH) is expensive and used in spacecraft air filters since hydroxide reacts with carbon dioxide, and lithium is lighter than sodium or potassium. Cesium and francium hydroxides are very expensive and linle used. Is this information consistent with your answer to the previous question? Information: Li,Na,K(= ions) vs. Li,Na,K(= metals) The use of a superscript zero after an clement (eg., Liv) indicates the element is in an uncharged metallic state (the zero oxidntion state). Li,Na, and K are all highly resctive metals we will occasionally encounter in this course-look for the zero superseript ()). If you don't see the zero superscript (") after L,Na, or K, assume it is an inert +1 counterion! 3. For each condensed formula, draw a line-bond sincture of the anion, and next to it draw the +1 counterion as a separate ion. NaOCH3NaHLi(CH2)3CH3LiN(CH3)2LiAlH4 Neither structure by itself tells the whole stoty. To get the most accurate picture of bicarbonate ion you must pverage ithe two different stractures. Figure 5.1: Bicarbonate ton (HCO3,) Critical Thinking Questions 4. A student looks at Model 2 and says she expects the bond between C and O2 to be halfway. between a single bond and a double bond (i.e., shorter and stronger than a single bond, but not as short and strong as a double bond) and that the same will be true of the bond between CandOr. Explain how she derived these conclusions from the information in Model 2 . 5. Based on the most accurate picture of bicarbonate ion, what is the charge on Ox ? Oy ? O7 ? (Note that it may be a fraction.) Briefly explain how you calculated your answers. 6. Organic chemists say that "the 1 charge on bicarbonate ion is "delocalized' between Ox and Oy." Come up with at least one synonym for the word "delocalized" in the preceding sentence. 7. On which do you expect to have a more intense and concentrated "hot spot" of negative charge: methoxide ion or bicarbonate ion? 8. Recall that one definition of a strong base is: "A molecule with a 1 formal charge localized on an H,C, N, or O atom. " Construct an explanation for why bicarbonate ion is NOT a strong base. 9. In which location is the Na counterion of baking soda (NaHCO3) most likely to reside: a) next to Ox b) next to Oy or c) halfway between Ox and Oy ? Briefly explain your reasoning. Model 3: Resonance Structures ChemActivity 5: Resonance Many ions (e.g., bicarbonate) bave charges that are delocalized (shared) among multiple atoms. To obtain an aceurate picture, we dratw all legitimate Lewis structares of ruch an ion and average them. Each of these legitimate Lewis structures is called a resonance structure (r.4.). To generate the full set of resonance structures for an ion: - Draw any one legitimate Lewis structare of the ion - CAREFULLY follow the rules for use of curved arrows to generate the other legitimate Lewis structures of the ion. (Each one will have the charge on a different atom in the ion). Memorization Task 5.1: Memorize Rules for Using the Three Types of Curved Arrows I. For anions: Always use Type 1&2 in tandem to move a charge (may use Type 3 with 1 \& 2). II. For cations: Use only. Type 3 (never 1 of 2) to move a 4 charge. III. You may not break any sigma bonds, move any atoms, or change the total number of electrons. IV. Fach C,N,D and X atom must have an octet (exception carbocations [R,C ] are legal). Anion Exampie: By ocavention: resoniace stricturs afe Hricketed together with weuare bracices and linked with a dopble-beaded antow. Cation Example: If you canhor legally move any charge (on any resonance structure) to generate a new structure, you are done. There ane no more resonance structurer in the set for that ion. Critical Thinking Questions 10. (E) Match each of the following descriptions to the correct type of curved arow (1,2, or 3): - A lone pair of electrons becomes a bond to an adjacent (nest-door) atom. - A pair of electrons in a bond slides down to become a lone pair on one atom in that bond. - A pair of electrons in a bond moves to become a bond to an adjacent atom









