Answered step by step
Verified Expert Solution
Question
1 Approved Answer
CHEMICAL ENGINEERING THERMODYNAMICS (b) Laboratory works were carried out to measure the composition of vapor and liquid of chloroform (1) and 1,4-dioxane (2) mixture. Vapor
CHEMICAL ENGINEERING THERMODYNAMICS 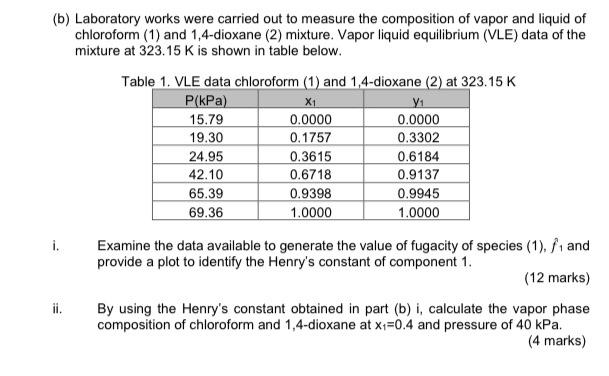
(b) Laboratory works were carried out to measure the composition of vapor and liquid of chloroform (1) and 1,4-dioxane (2) mixture. Vapor liquid equilibrium (VLE) data of the mixture at 323.15 K is shown in table below. Table 1. VLE data chloroform (1) and 1,4-dioxane (2) at 323.15 K P(kPa) X1 Y1 15.79 0.0000 0.0000 19.30 0.1757 0.3302 24.95 0.3615 0.6184 42.10 0.6718 0.9137 65.39 0.9398 0.9945 69.36 1.0000 1.0000 i. Examine the data available to generate the value of fugacity of species (1). f1 and provide a plot to identify the Henry's constant of component 1. (12 marks) By using the Henry's constant obtained in part (b) i, calculate the vapor phase composition of chloroform and 1,4-dioxane at x1=0.4 and pressure of 40 kPa. (4 marks) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


