Chemical equation mateial?
physical properties?
Theortical?
Actual=3
% yield?
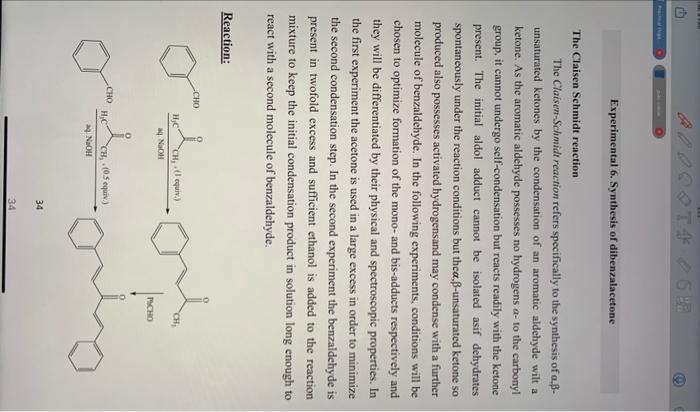
The Claisen Sehmidt reaction The Claisen-Schmidt reaction refers specifically to the synthesis of , unsaturated ketones by the condensation of an aromatic aldehyde wilt a ketone. As the aromatic aldehyde possesses no hydrogens a - to the carbonyl group, it cannot undergo self-condensation but reacts readily with the ketone present. The initial aldol adduct cannot be isolated asif dehydrates spontaneously under the reaction conditions but the ,-unsaturated ketone so produced also possesses activated hydrogensand may condense with a further molecule of benzaldehyde. In the following experiments, conditions will be chosen to optimize formation of the mono- and bis-adducts respectively and they will be differentiated by their physical and spectroscopic properties. In the first experiment the acetone is used in a large excess in order to minimize the second condensation step. In the second experiment the benzaldehyde is present in twofold excess and sufficient ethanol is added to the reaction mixture to keep the initial condensation product in solution long enough to react with a second molecule of benzaldehyde. Reaction: 34 34 The Claisen Schmidt reaceien The C Taines-Schmst foucrion rfers specifically to the synthesis of affunsaturatnd hetones by the condensation of an aromatic aldelyde wilt a kctoec. As the aromatic aldelyde possesies Bo lrydrogens an to the carbany! grouph if cannol undergo seff-eondensation but reacts readily with the kctiene preseer. The initial aldod adduct canoot be isolated asif dehydrates Fpoctanceucly under the raction conditions hut thea ff-ussaturated ketcse so peoduced alwo possesises acsivased hydrogensand may condense wath a further molecule of benralschyde. In the following experiments, conditions wilt be choren to optimine foemation of the mopor and bes-addacts respectively and they will be differentiated by their physied and spectroscopic propertics. In the first experiment the acetone is used in a large excens in erder to manimioe the second condensation sep. In the second experiment the benraliehyde is present in twofold excess and saficient cthanol is added to the reaction mixture to keep the initial coeldericatiee problact in solution loee enough to react with a recond molocule of bcevalisehyde. Reactions Materials. 1.Benzaldehyde2.Acetone3.NaOS!5.2ml2ml5gm Procrifure: 1. Awell stirted solution of 5 am of sectiam hydroxide in 50 mil aater aisif 40 ml ethanol wascoolel at 20C. 2-Stir vigerously and asded oee half of the previoully ptepared mincture to (benzaldehyde 5.2ml and acetone 2ml ) 3. Continoe the iairring after 15 min. add the remainder of the abon t mixture 4. Caerinse stakiry for a further 30 mia. 4. The precipitate is formod, filtered off, washed wath cold water, dnced. tecrystalfiasd fiom ethinol. The yield of the protact 5.4 en ( (koe, mp1124c








