Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chemical Mass Balance 4.78. Ethylene oxide is produced by the catalytic oxidation of ethylene: 2C2H4+O22C2H4O An undesired competing reaction is the combustion of ethylene: C2H4+3O22CO2+2H2O
Chemical Mass Balance 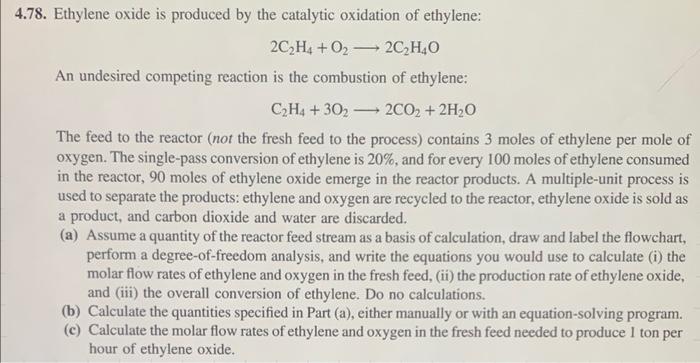
4.78. Ethylene oxide is produced by the catalytic oxidation of ethylene: 2C2H4+O22C2H4O An undesired competing reaction is the combustion of ethylene: C2H4+3O22CO2+2H2O The feed to the reactor (not the fresh feed to the process) contains 3 moles of ethylene per mole of oxygen. The single-pass conversion of ethylene is 20%, and for every 100 moles of ethylene consumed in the reactor, 90 moles of ethylene oxide emerge in the reactor products. A multiple-unit process is used to separate the products: ethylene and oxygen are recycled to the reactor, ethylene oxide is sold as a product, and carbon dioxide and water are discarded. (a) Assume a quantity of the reactor feed stream as a basis of calculation, draw and label the flowchart, perform a degree-of-freedom analysis, and write the equations you would use to calculate (i) the molar flow rates of ethylene and oxygen in the fresh feed, (ii) the production rate of ethylene oxide, and (iii) the overall conversion of ethylene. Do no calculations. (b) Calculate the quantities specified in Part (a), either manually or with an equation-solving program. (c) Calculate the molar flow rates of ethylene and oxygen in the fresh feed needed to produce 1 ton per hour of ethylene oxide 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


