Answered step by step
Verified Expert Solution
Question
1 Approved Answer
CHEMICAL REACTION ENGINEERING (I) 1) The esterification reaction between acetic acid and ethyl alcohol will be carried out in a continuously stirred semi-batch tank reactor
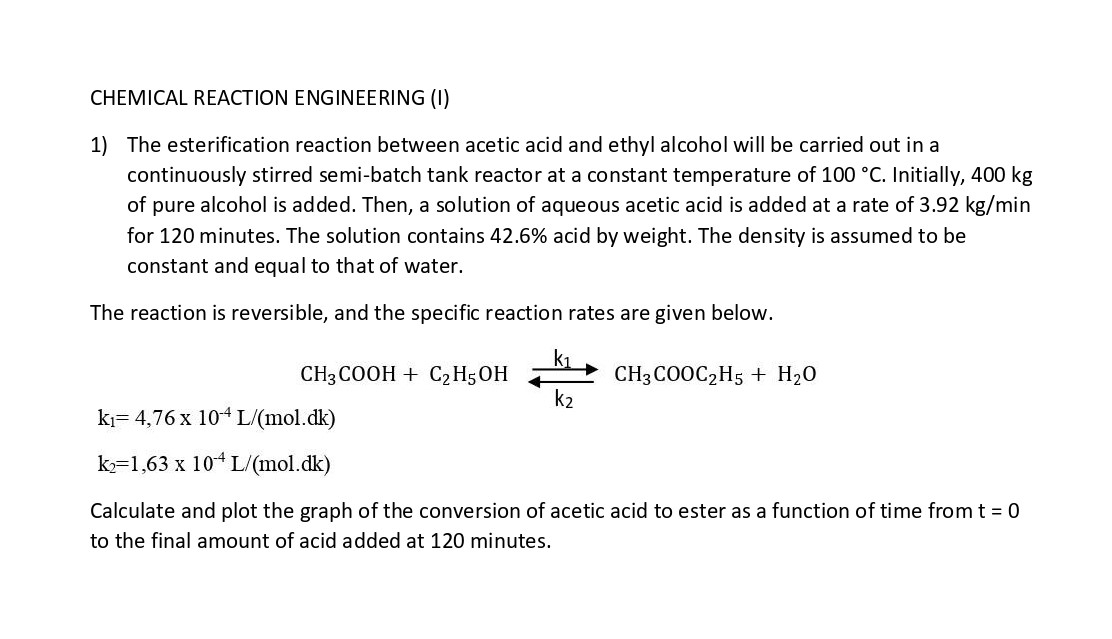 CHEMICAL REACTION ENGINEERING (I) 1) The esterification reaction between acetic acid and ethyl alcohol will be carried out in a continuously stirred semi-batch tank reactor at a constant temperature of 100C. Initially, 400kg of pure alcohol is added. Then, a solution of aqueous acetic acid is added at a rate of 3.92kg/min for 120 minutes. The solution contains 42.6% acid by weight. The density is assumed to be constant and equal to that of water. The reaction is reversible, and the specific reaction rates are given below. CH3COOH+C2H5OHk2k1CH3COOC2H5+H2Ok1=4,76104L/(mol.dk)k2=1,63104L/(mol.dk) Calculate and plot the graph of the conversion of acetic acid to ester as a function of time from t=0 to the final amount of acid added at 120 minutes
CHEMICAL REACTION ENGINEERING (I) 1) The esterification reaction between acetic acid and ethyl alcohol will be carried out in a continuously stirred semi-batch tank reactor at a constant temperature of 100C. Initially, 400kg of pure alcohol is added. Then, a solution of aqueous acetic acid is added at a rate of 3.92kg/min for 120 minutes. The solution contains 42.6% acid by weight. The density is assumed to be constant and equal to that of water. The reaction is reversible, and the specific reaction rates are given below. CH3COOH+C2H5OHk2k1CH3COOC2H5+H2Ok1=4,76104L/(mol.dk)k2=1,63104L/(mol.dk) Calculate and plot the graph of the conversion of acetic acid to ester as a function of time from t=0 to the final amount of acid added at 120 minutes Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


