Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chemical Reactions: Molecular Equation: HCl(aq) + NaOH(aq) Net Ionic Equation: H+(aq) + OH(aq) Details Trial 1 Molarity of HCl Standard Solution Volume (mL) HCl
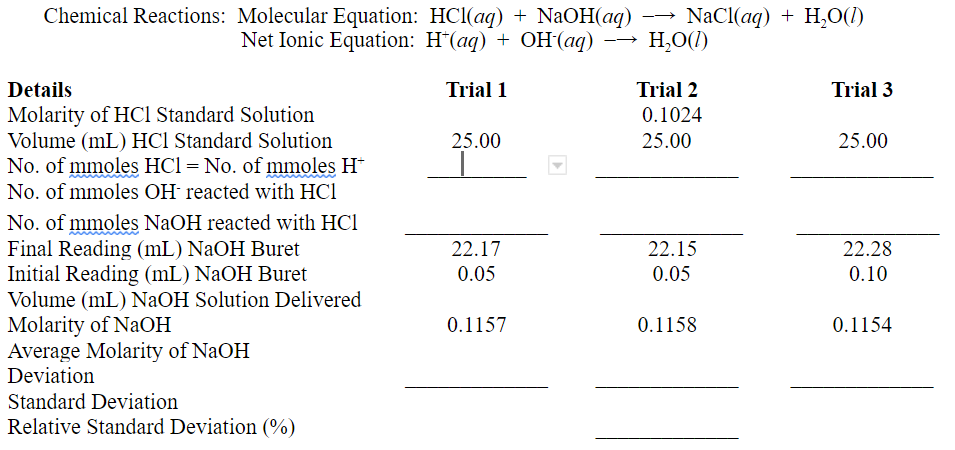
Chemical Reactions: Molecular Equation: HCl(aq) + NaOH(aq) Net Ionic Equation: H+(aq) + OH(aq) Details Trial 1 Molarity of HCl Standard Solution Volume (mL) HCl Standard Solution No. of mmoles HCl = No. of mmoles H* 25.00 NaCl(aq) + H2O(1) HO(1) Trial 2 0.1024 25.00 Trial 3 25.00 No. of mmoles OH reacted with HC1 No. of mmoles NaOH reacted with HCI Final Reading (mL) NaOH Buret 22.17 22.15 22.28 Initial Reading (mL) NaOH Buret 0.05 0.05 0.10 Volume (mL) NaOH Solution Delivered Molarity of NaOH 0.1157 0.1158 0.1154 Average Molarity of NaOH Deviation Standard Deviation Relative Standard Deviation (%)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
It appears from the information provided that you have carried out a titration experiment in which y...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


