Question
10) By using the given 1) electronic absorption spectrum, 2) the Tanabo-Sugano diagram for d complex in octahedral ligand field and 3) the absorption/emission
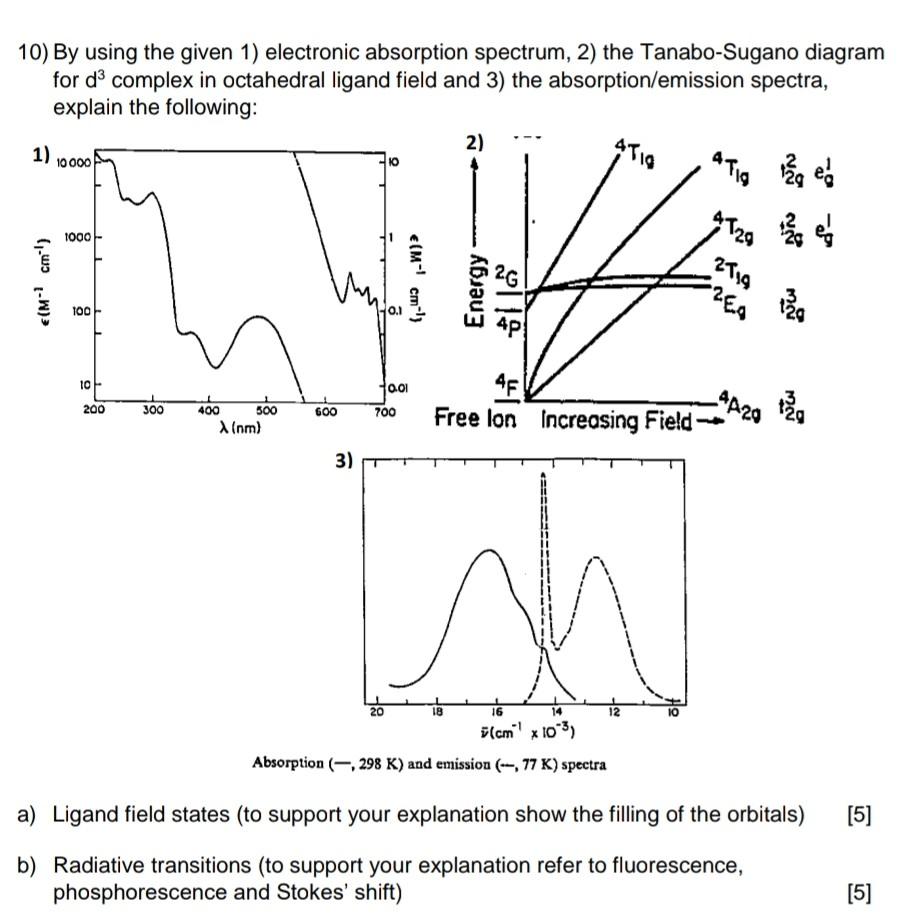
10) By using the given 1) electronic absorption spectrum, 2) the Tanabo-Sugano diagram for d complex in octahedral ligand field and 3) the absorption/emission spectra, explain the following: 1) 10000 (M- cm) 1000 100 10 200 300 400 500 X (nm) 600 3) 700 20 (M- cm-} 0.01 2) 18 Energy - 4719 16 14 (cm x 10) Absorption (-,298 K) and emission (-, 77 K) spectra 4T19 AF Free lon Increasing Field- 12 4729 2719 -4A29 12 e 1 13g tg a) Ligand field states (to support your explanation show the filling of the orbitals) [5] b) Radiative transitions (to support your explanation refer to fluorescence, phosphorescence and Stokes' shift) [5]
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Solution a Ligand field theory explains orbital arrangements ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
11th edition
1118133579, 978-1118133576
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



