Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The figure shows the solubility of a compound A in three different solvents (I-III) as a function of temperature. Which solvent would you choose
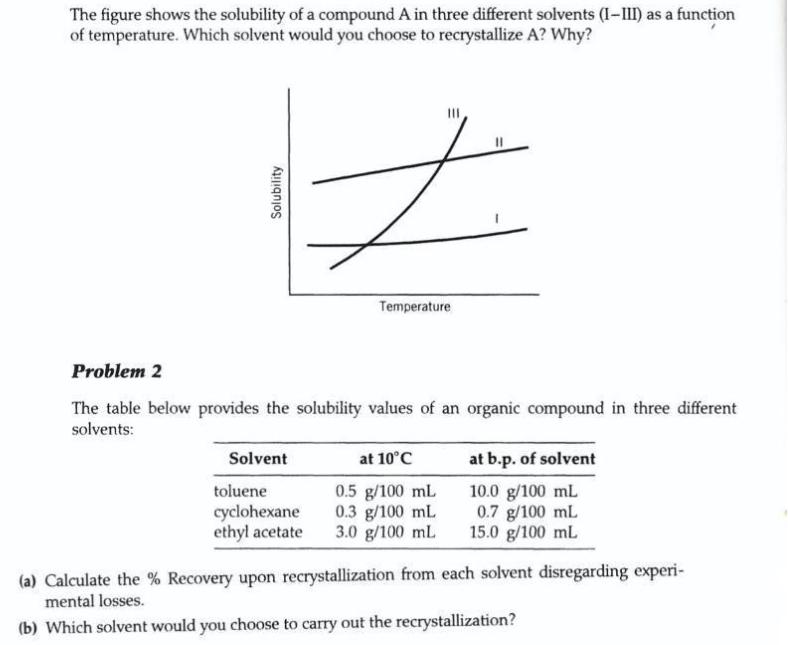
The figure shows the solubility of a compound A in three different solvents (I-III) as a function of temperature. Which solvent would you choose to recrystallize A? Why? Temperature Problem 2 The table below provides the solubility values of an organic compound in three different solvents: Solvent at 10C at b.p. of solvent toluene cyclohexane ethyl acetate 0.5 g/100 mL 0.3 g/100 mL 3.0 g/100 mL 10.0 g/100 mL 0.7 g/100 mL 15.0 g/100 mL (a) Calculate the % Recovery upon recrystallization from each solvent disregarding experi- mental losses. (b) Which solvent would you choose to carry out the recrystallization? Solubility
Step by Step Solution
★★★★★
3.36 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Ans Crystallization is the process of purification of impure sample in which sample is dissolved in ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


