Question
9. The kinetics of the oxidation of ferrodoxin by oxygen were investigated in a careful series of experiments, shown below at 25 C. 2
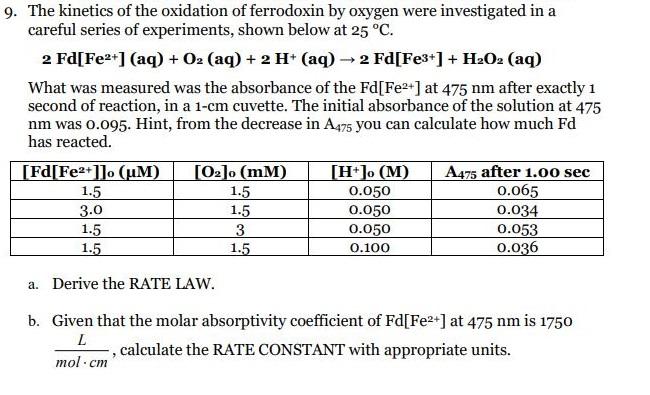
9. The kinetics of the oxidation of ferrodoxin by oxygen were investigated in a careful series of experiments, shown below at 25 C. 2 Fd[Fe2*] (aq) + O2 (aq) + 2 H* (aq) 2 Fd[Fe3*] + H:O2 (aq) What was measured was the absorbance of the Fd[Fe2+] at 475 nm after exactly 1 second of reaction, in a 1-cm cuvette. The initial absorbance of the solution at 475 nm was 0.095. Hint, from the decrease in A475 you can calculate how much Fd has reacted. [Fd[Fe2+]]o (uM) [02]o (mM) [H*]o (M) A475 after 1.0o sec 0.065 0.034 0.053 0.036 1.5 1.5 1.5 3. 1.5 0.050 3.0 1.5 1.5 0.050 0.050 0.100 a. Derive the RATE LAW. b. Given that the molar absorptivity coefficient of Fd[Fe2+] at 475 nm is 1750 , calculate the RATE CONSTANT with appropriate units. mol -
Step by Step Solution
3.37 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction To Statistics And Data Analysis
Authors: Roxy Peck, Chris Olsen, Tom Short
6th Edition
1337793612, 978-1337793612
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



