Answered step by step
Verified Expert Solution
Question
1 Approved Answer
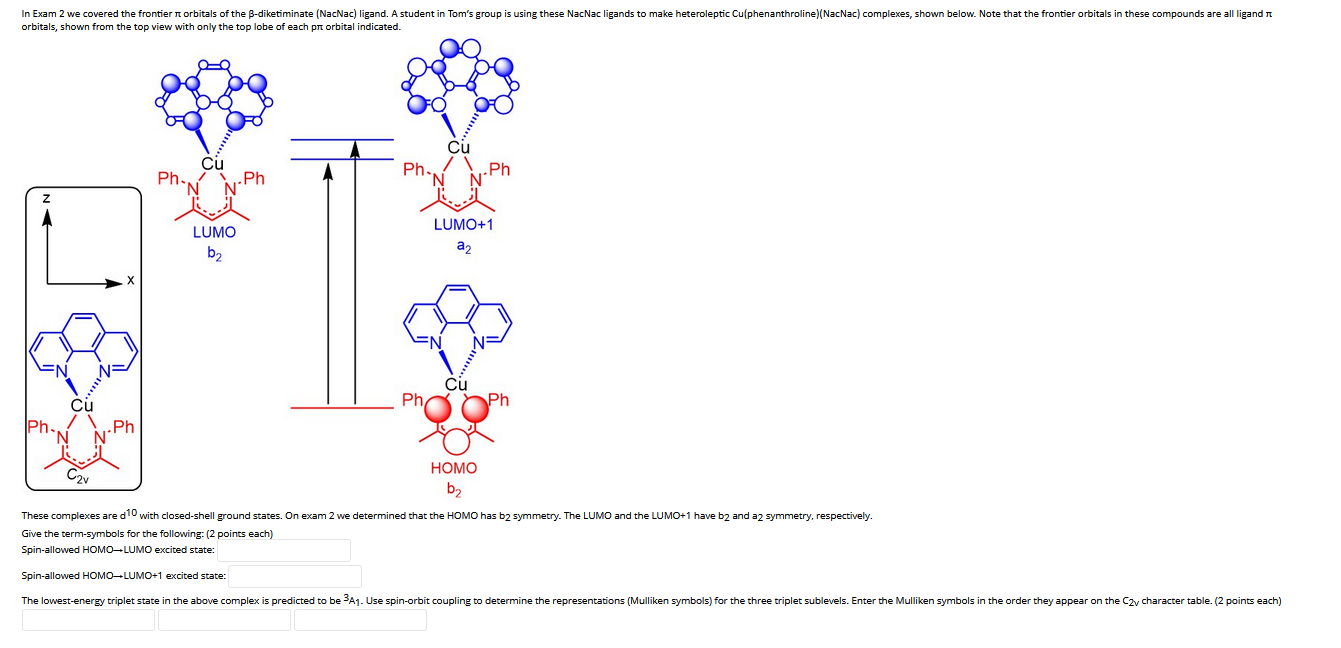
In Exam 2 we covered the frontier orbitals of the B-diketiminate (NacNac) ligand. A student in Tom's group is using these NacNac ligands to
In Exam 2 we covered the frontier orbitals of the B-diketiminate (NacNac) ligand. A student in Tom's group is using these NacNac ligands to make heteroleptic Cu(phenanthroline) (NacNac) complexes, shown below. Note that the frontier orbitals in these compounds are all ligand orbitals, shown from the top view with only the top lobe each pr orbital indicated. Ph. .Ph Ph- Cu LUMO b .Ph Ph Cu LUMO+1 a Cu .Ph HOMO b Ph These complexes are d10 with closed-shell ground states. On exam 2 we determined that the HOMO has b2 symmetry. The LUMO and the LUMO+1 have b2 and a2 symmetry, respectively. Give the term-symbols for the following: (2 points each) Spin-allowed HOMO-LUMO excited state: Spin-allowed HOMO-LUMO+1 excited state: The lowest-energy triplet state in the above complex is predicted to be 3A. Use spin-orbit coupling to determine the representations (Mulliken symbols) for the three triplet sublevels. Enter the Mulliken symbols in the order they appear on the C2y character table. (2 points each)
Step by Step Solution
★★★★★
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
F21 58 cos 30 5023 kN Fyl 58 sin 30 29 kN F2250 cos 45 3536 kN F2 50 sin 45 3536 kN F ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started