Question
A water sample was analyzed and was found to have the following constituents: Ca, mg/L HCO,, mg/L 82 so,, mg/L 122 112 123 Mg,
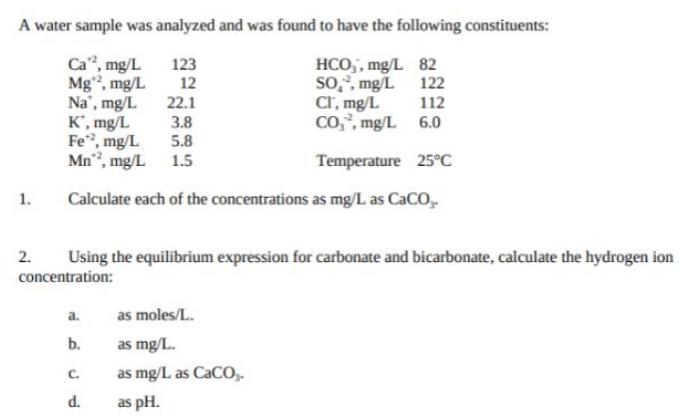
A water sample was analyzed and was found to have the following constituents: Ca", mg/L HCO,, mg/L 82 so,, mg/L 122 112 123 Mg, mg/L 22.1 12 Na', mg/L K', mg/L Fe", mg/L Mn", mg/L 1.5 Cr, mg/L Co, mg/L 6.0 3.8 5.8 Temperature 25C 1. Calculate each of the concentrations as mg/L as CaCO,. 2. Using the equilibrium expression for carbonate and bicarbonate, calculate the hydrogen ion concentration: a. as moles/L. b. as mg/L. C. as mg/L as CaCO,. d. as pH.
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Corporate Finance
Authors: Berk, DeMarzo, Harford
2nd edition
132148234, 978-0132148238
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



