Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An experiment was carried out to investigate the effect of concentration on the reaction: BrO3 (aq) + 5 Br (aq) + 6 H* (aq)
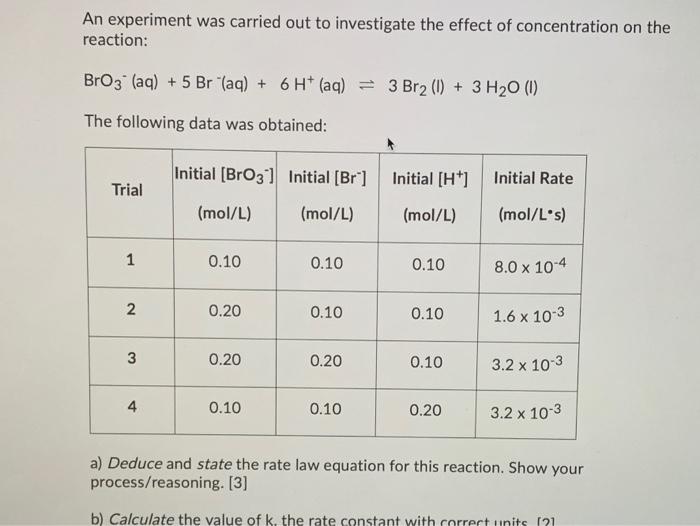
An experiment was carried out to investigate the effect of concentration on the reaction: BrO3 (aq) + 5 Br "(aq) + 6 H* (aq) = 3 Br2 (1) + 3 H20 (I) The following data was obtained: Initial [BrO3] Initial [Br] Initial [H*] Initial Rate Trial (mol/L) (mol/L) (mol/L) (mol/L's) 1 0.10 0.10 0.10 8.0 x 10-4 2 0.20 0.10 0.10 1.6 x 10-3 3 0.20 0.20 0.10 3.2 x 10-3 4 0.10 0.10 0.20 3.2 x 10-3 a) Deduce and state the rate law equation for this reaction. Show your process/reasoning. [3] b) Calculate the value of k. the rate constant with correct units (21
Step by Step Solution
★★★★★
3.54 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6363b28c09fb5_238675.pdf
180 KBs PDF File
6363b28c09fb5_238675.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


