Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate charged and uncharged ration of weak acid and weak base drug under normal and basic urine pH. What is the effect of urine
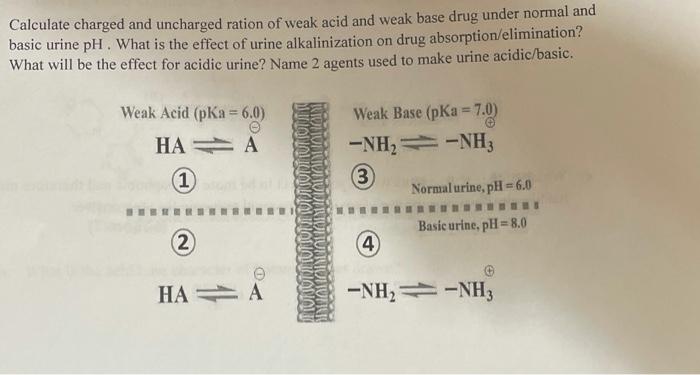
Calculate charged and uncharged ration of weak acid and weak base drug under normal and basic urine pH. What is the effect of urine alkalinization on drug absorption/elimination? What will be the effect for acidic urine? Name 2 agents used to make urine acidic/basic. Weak Acid (pKa = 6.0) Weak Base (pKa = 7.0) !! HA A -NH,= -NH3 3 (1) Normal urine, pH= 6.0 Basic urine, pH= 8.0 2) 4) HA = A -NH, -NH3
Step by Step Solution
★★★★★
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
Most drugs are ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
636871154cd7d_241906.pdf
180 KBs PDF File
636871154cd7d_241906.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


