Question
20-21. Standard addition. To measure Ca in breakfast cereal, 0.521 6 g of crushed Cheerios was ashed in a crucible at 600C in air
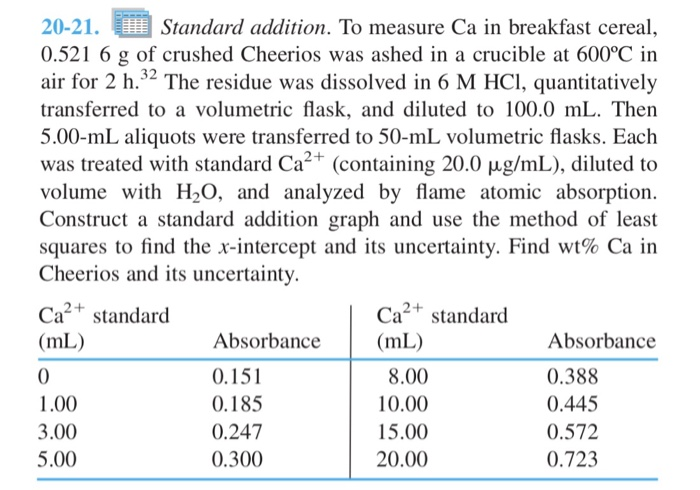
20-21. Standard addition. To measure Ca in breakfast cereal, 0.521 6 g of crushed Cheerios was ashed in a crucible at 600C in air for 2 h.32 The residue was dissolved in 6 M HCl, quantitatively transferred to a volumetric flask, and diluted to 100.0 mL. Then 5.00-mL aliquots were transferred to 50-mL volumetric flasks. Each was treated with standard Ca+ (containing 20.0 g/mL), diluted to volume with HO, and analyzed by flame atomic absorption. Construct a standard addition graph and use the method of least squares to find the x-intercept and its uncertainty. Find wt% Ca in Cheerios and its uncertainty. Ca+ standard (mL) 0 1.00 3.00 5.00 Absorbance 0.151 0.185 0.247 0.300 Ca+ standard (mL) 8.00 10.00 15.00 20.00 Absorbance 0.388 0.445 0.572 0.723
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
Value of X interce...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantitative Chemical Analysis
Authors: Daniel C. Harris
8th edition
1429218150, 978-1429218153
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



