Question
Chemistry equation 4: (iv) Calculate, with the help of benzene's density, the mass of the volume benzene used. Calculate the molar mass of naphthalene by
Chemistry

equation 4:
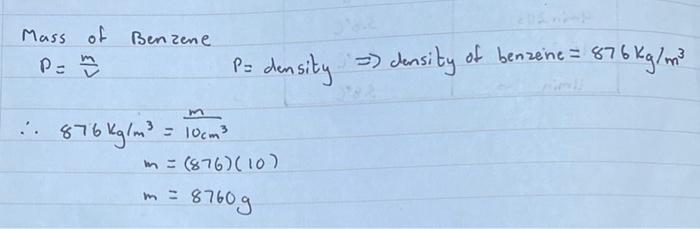
(iv) Calculate, with the help of benzene's density, the mass of the volume benzene used. Calculate the molar mass of naphthalene by making use of equation (4).
Step by Step Solution
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
a density 876 Kg m3 1 kg 1000g im ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Finance Applications and Theory
Authors: Marcia Cornett, Troy Adair
3rd edition
1259252221, 007786168X, 9781259252228, 978-0077861681
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



