Question
Chemistry HomdinalS y Tame lft ot Quen A factory has a unique product based on the following equation: 2A -R, ke =12 Lmor'min (2nd order).
Chemistry
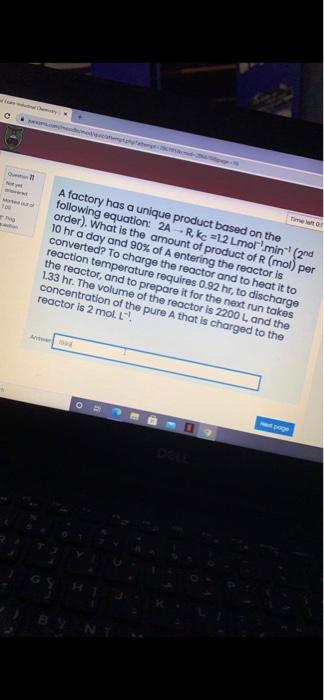
HomdinalS y Tame lft ot Quen A factory has a unique product based on the following equation: 2A -R, ke =12 Lmor'min (2nd order). What is the amount of product of R (mol) per 10 hr a day and 90% of A entering the reactor is converted? To charge the reactor and to heat it to reaction temperature requires 0.92 hr, to discharge the reactor, and to prepare it for the next run takes 1.33 hr. The volume of the reactor is 2200 L and the concentration of the pure A that is charged to the reactor is 2 mol. L. Ante Tpoge 13 DELE
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cost management a strategic approach
Authors: Edward J. Blocher, David E. Stout, Gary Cokins
5th edition
73526940, 978-0073526942
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



