Question
The compound BaTiO 3 has a perovskite structure. Considering the following ratio of radii: (R (O 2- ) = 0.146nm, R (Ti 4+ ) =
The compound BaTiO3 has a perovskite structure. Considering the following ratio of radii: (R (O2-) = 0.146nm, R (Ti4+ ) = 0.071nm, R (Ba2+) = 0.113nm, Na = 6.023x1023 mol-1, Patm (Ba) = 137.33 g / mol ; Patm (Ti) = 47.88 g / mol; Patm (O) = 15.99 g / mol;
b) Determine the lattice parameters of the unit cell
c) Determine the planar density in the (101) plane (in ions / nm2)
d) Determine the volumetric density (in g / cm3)
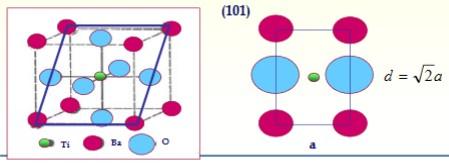
Ti Ba (101) d = 2a
Step by Step Solution
3.53 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Answer A 0 R Ba Patm Ba Patm Ti Answer 0146 AM 0113nm 4788 g...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Probability
Authors: Mark Daniel Ward, Ellen Gundlach
1st edition
716771098, 978-1319060893, 1319060897, 978-0716771098
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



