Question
What is the limiting reactant? How can you tell based on your observations? Using the table provided in appendix H, look up the solubility

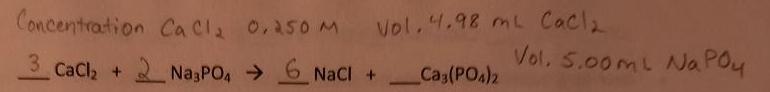
What is the limiting reactant? How can you tell based on your observations? Using the table provided in appendix H, look up the solubility values for the two products NaCl: Ca3(PO4)2 Based on the solubility numbers, what would the solid product be? Concentration CaCla 0,250 vol. 4.98 mL Cacl2 Vol. 5.00mL Na Poy 3 CaCl, + 2Na3PO, 6 NaCI + Cas(PO)2
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
What is the Limiting Reactant The limiting reagent is the reactant that is used up completely This stops the reaction and no further products are made ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Exploring Management
Authors: John R. Schermerhorn, Daniel G. Bachrach
5th edition
978-1119117742, 1119140293, 1119117747, 9781119140290, 978-1119231936
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



