Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chemistry Question 1 According to the experiments, the change in entropy of 1 5 0 g of water when heated from an unknown temperature to
Chemistry
Question
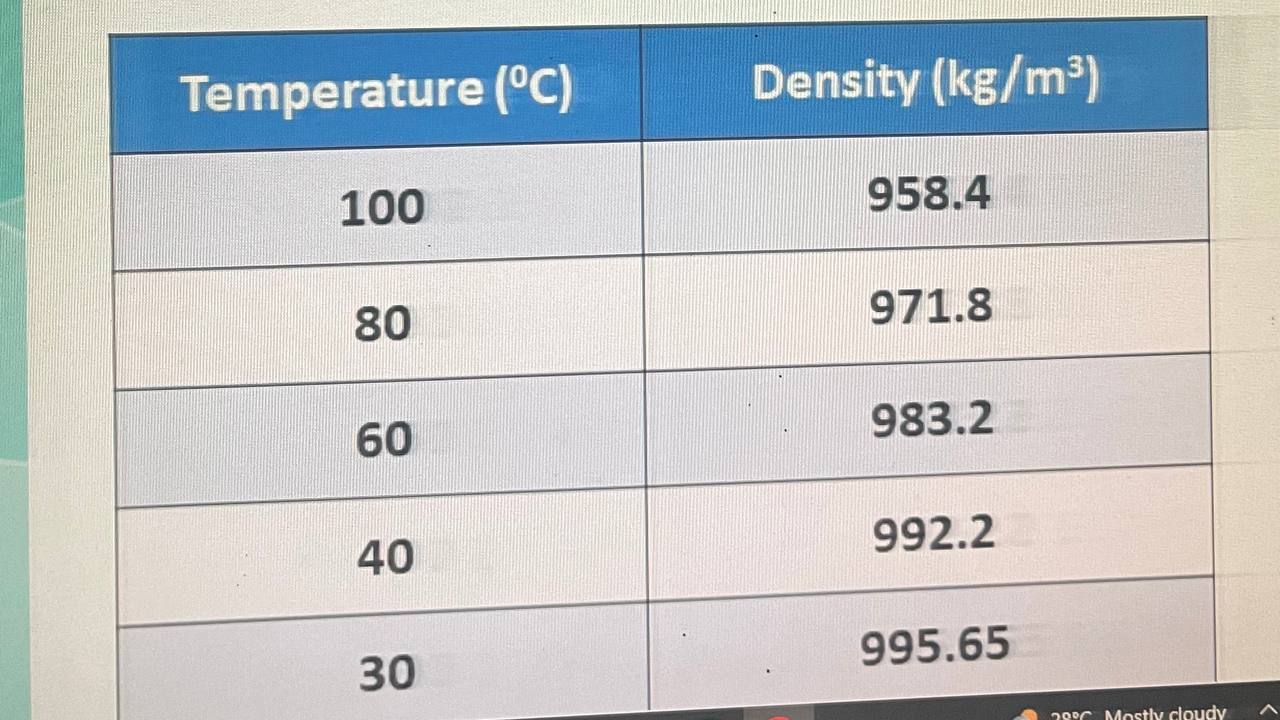
According to the experiments, the change in entropy of g of water when heated from an unknown temperature to deg C at constant pressure is JK Cp of water, assumed to be constant throughout the process, is JgK Determine the initial density of water in kgm before it was being heated. Density of water at different temperatures is presented use this table only:
Answer Format: decimals
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


