Answered step by step
Verified Expert Solution
Question
1 Approved Answer
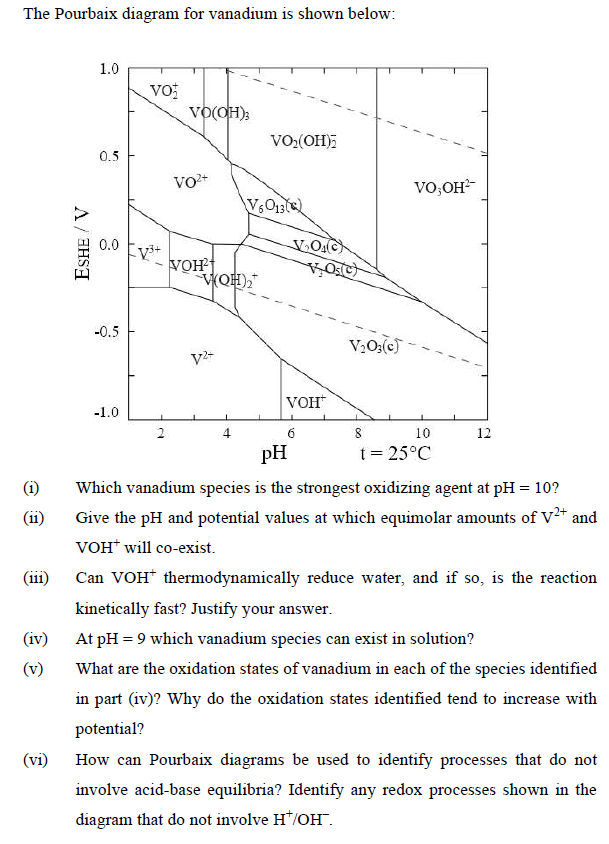
The Pourbaix diagram for vanadium is shown below: (11) (111) (iv) (vi) ESHE / V 1.0 0.5 0.0 -0.5 -1.0 Vo -V+ VO(OH); VO+
The Pourbaix diagram for vanadium is shown below: (11) (111) (iv) (vi) ESHE / V 1.0 0.5 0.0 -0.5 -1.0 Vo -V+ VO(OH); VO+ VOH V(OH) V2+ VO(OH) V6013 VO(c) Os(C) VOH VO3(c) VO,OH 8 10 t = 25C 12 pH Which vanadium species is the strongest oxidizing agent at pH = 10? Give the pH and potential values at which equimolar amounts of V+ and VOH will co-exist. Can VOH* thermodynamically reduce water, and if so, is the reaction kinetically fast? Justify your answer. At pH = 9 which vanadium species can exist in solution? What are the oxidation states of vanadium in each of the species identified in part (iv)? Why do the oxidation states identified tend to increase with potential? How can Pourbaix diagrams be used to identify processes that do not involve acid-base equilibria? Identify any redox processes shown in the diagram that do not involve H/OH".
Step by Step Solution
★★★★★
3.47 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Answer strongest oxidizing agent at ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started