Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The resistances of CH3COOCI3 solutions of various concentrations measured at 25 C using a conductivity cell with a cell constant of 0.2063 cm-1 are
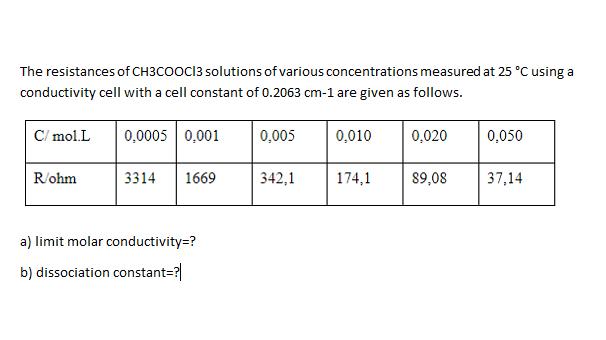
The resistances of CH3COOCI3 solutions of various concentrations measured at 25 C using a conductivity cell with a cell constant of 0.2063 cm-1 are given as follows. C/ mol.L 0,0005 0,001 0,005 0,010 0,020 0,050 R/ohm 3314 1669 342,1 174,1 89,08 37,14 a) limit molar conductivity=? b) dissociation constant=?
Step by Step Solution
★★★★★
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
a Limit Molar Conductivity Cell constant ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6363c30f3e501_238694.pdf
180 KBs PDF File
6363c30f3e501_238694.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


