Question
Given below the half reaction of certain specles with E vs SCE, whlch one of them can't be determined using polarography method or technique
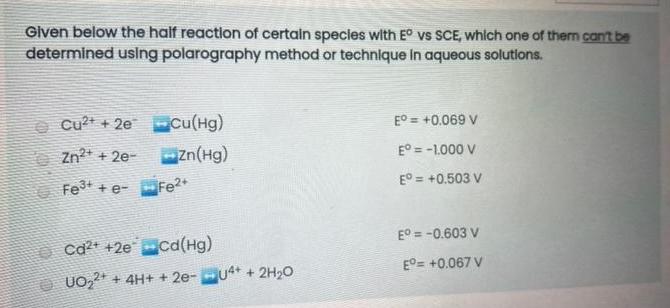
Given below the half reaction of certain specles with E vs SCE, whlch one of them can't be determined using polarography method or technique In aqueous solutions. O cu?t + 2e cu(Hg) E = +0.069 V %3! O Zn?* + 2e- Zn(Hg) E = -1.000 V Fet + e- Fe2 E = +0.503 V O ca* +2e ca(Hg) E = -0.603 V E= +0.067 V O UO,2* + 4H+ + 2e-u4* + 2H,O
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Francis A. Carey
4th edition
0072905018, 978-0072905014
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



