Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What are the concentrations of [K*), [OH], [CO32] and (H*), in a 1.2 M solution of K2CO3 ? (Note: Question is asking for concentrations
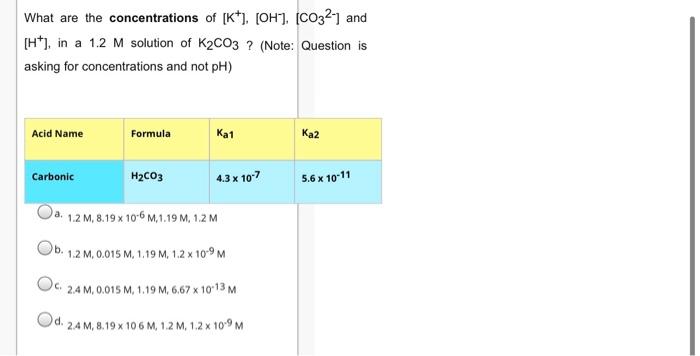

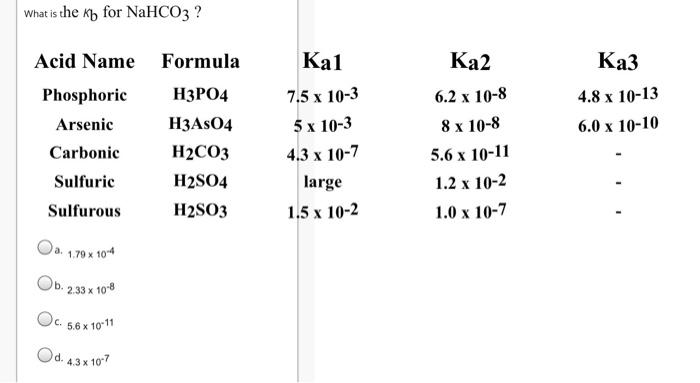


What are the concentrations of [K*), [OH], [CO32] and (H*), in a 1.2 M solution of K2CO3 ? (Note: Question is asking for concentrations and not pH) Acid Name Formula Ka1 Ka2 Carbonic H2CO3 4.3 x 107 5.6 x 1011 a. 1.2 M, 8.19 x 106 M,1.19 M, 1.2 M Ob. 1.2 M, 0.015 M, 1.19 M, 1.2 x 10-9 M Oc. C. 2.4 M, 0.015 M, 1.19 M, 6.67 x 1013 M Od. 2.4 M, 8.19 x 10 6 M, 1.2 M, 1.2 x 10-9 M Choose the correct equilibrium chemical equation for the Kb of HASO42- Oa. H3ASO4 + H20 = H3o* + H2ASO4 Ob. H2ASO4" + H20 at OH" + H3ASO4 Oc. C. H2ASO4" + H20 H3o* + HASO42 Od. HASO42 + H20 = OH" + H2ASO4 e. HASO42+ H20 = H30* + AsO43 What is the kb for NaHCO3 ? Acid Name Formula Kal 2 Phosphoric 4 7.5 x 10-3 6.2 x 10-8 4.8 x 10-13 Arsenic H3ASO4 5 x 10-3 8 x 10-8 6.0 x 10-10 Carbonic H2CO3 4.3 x 10-7 5.6 x 10-11 Sulfuric H2SO4 large 1.2 x 10-2 Sulfurous H2SO3 1.5 x 10-2 1.0 x 10-7 a. 1.79 x 104 2.33 x 108 Oc. C. 5.6 x 10 11 Od. 43x 107 Which of the following is a polyprotic acid ? Oa. a. NaHCO3 Ob. NaOH C. H2SO3 Od. HNO3 Which of the following is an ampholyte Oa. HCO3 Ob. b. HASO42- Oc. C. HSO3 Od. H2PO4 Oe. All of the above
Step by Step Solution
★★★★★
3.25 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Answers 1 d 2 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
63628789abcca_236198.pdf
180 KBs PDF File
63628789abcca_236198.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


