Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chemoselectivity in functional group transformation is a coveted outcome in organic synthesis. This is commonly achieved by exploiting unique characteristics inherent in closely related

![]()
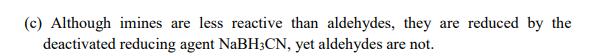
![]()
![]()
Chemoselectivity in functional group transformation is a coveted outcome in organic synthesis. This is commonly achieved by exploiting unique characteristics inherent in closely related reagents. Refer to the observations noted below and advance reasons behind the observed outcomes: (You may use appropriate illustrations or mechanisms, but in either case, be brief) (2 Marks Each) (a) Resorcinol (benzene-1,3-diol) readily undergoes hydrogenation to cyclohexane- 1,3-dione in the presence of H/Ni, but benzene does not. (b) PCC oxidizes 1-methylcyclopent-2-enol, but not 1-methylcyclopent-3-enol. (c) Although imines are less reactive than aldehydes, they are reduced by the deactivated reducing agent NaBH3CN, yet aldehydes are not. (d) MCPBA epoxidizes electron-rich alkenes, while HO/NaOH epoxidizes electron-deficient alkenes. (e) Unstabilized phosphorus ylides react with aldehydes to provide Z-alkenes, yet stabilized phosphorus ylides react to provide E-alkenes.
Step by Step Solution
★★★★★
3.36 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


