Answered step by step
Verified Expert Solution
Question
1 Approved Answer
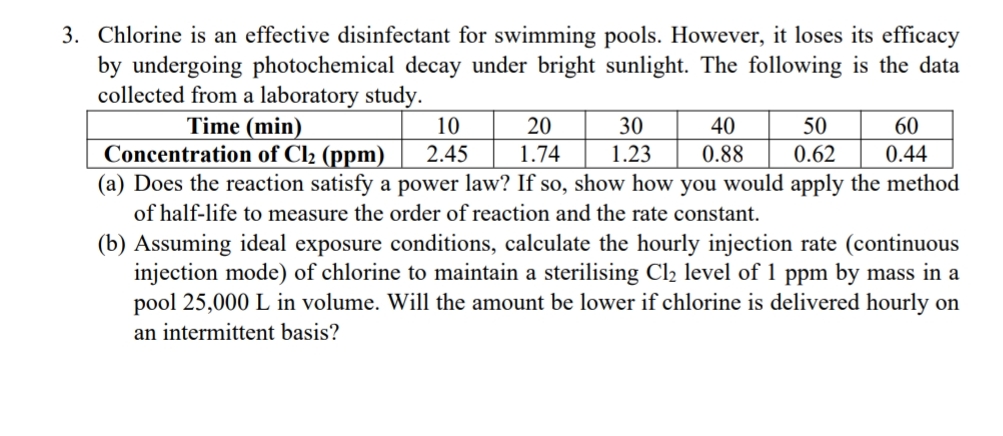
Chlorine is an effective disinfectant for swimming pools. However, it loses its efficacy by undergoing photochemical decay under bright sunlight. The following is the data
Chlorine is an effective disinfectant for swimming pools. However, it loses its efficacy by undergoing photochemical decay under bright sunlight. The following is the data collected from a laboratory study.
tableTime minConcentration of
a Does the reaction satisfy a power law? If so show how you would apply the method of halflife to measure the order of reaction and the rate constant.
b Assuming ideal exposure conditions, calculate the hourly injection rate continuous injection mode of chlorine to maintain a sterilising level of by mass in a pool L in volume. Will the amount be lower if chlorine is delivered hourly on an intermittent basis?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


