Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chooze magazine tested a popular brand of antacid and compared the results with Grandma's antacid remedy namely baking soda. The experimental procedure followed by
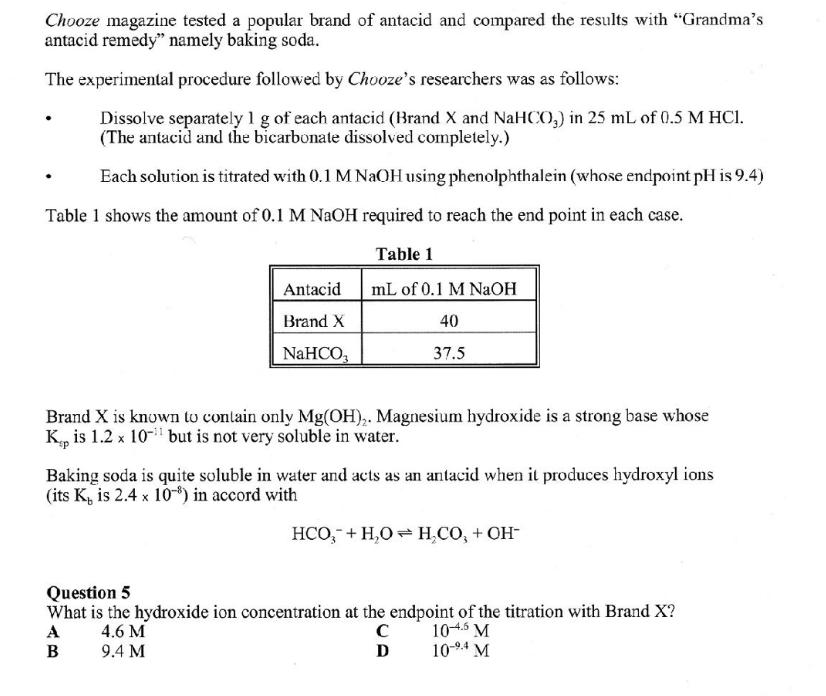
Chooze magazine tested a popular brand of antacid and compared the results with "Grandma's antacid remedy" namely baking soda. The experimental procedure followed by Chooze's researchers was as follows: Dissolve separately 1 g of each antacid (Brand X and NaHCO3) in 25 mL of 0.5 M HCI. (The antacid and the bicarbonate dissolved completely.) Each solution is titrated with 0.1 M NaOH using phenolphthalein (whose endpoint pH is 9.4) Table 1 shows the amount of 0.1 M NaOH required to reach the end point in each case. Table 1 mL of 0.1 M NaOH 40 37.5 Antacid Brand X NaHCO, Brand X is known to contain only Mg(OH),. Magnesium hydroxide is a strong base whose K is 1.2 x 10 but is not very soluble in water. Baking soda is quite soluble in water and acts as an antacid when it produces hydroxyl ions (its K, is 2.4 x 10) in accord with HCO + HO HCO, + OH- Question 5 What is the hydroxide ion concentration at the endpoint of the titration with Brand X? A 4.6 M 104.5 M B 9.4 M D 10-4 M
Step by Step Solution
★★★★★
3.39 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


