Answered step by step
Verified Expert Solution
Question
1 Approved Answer
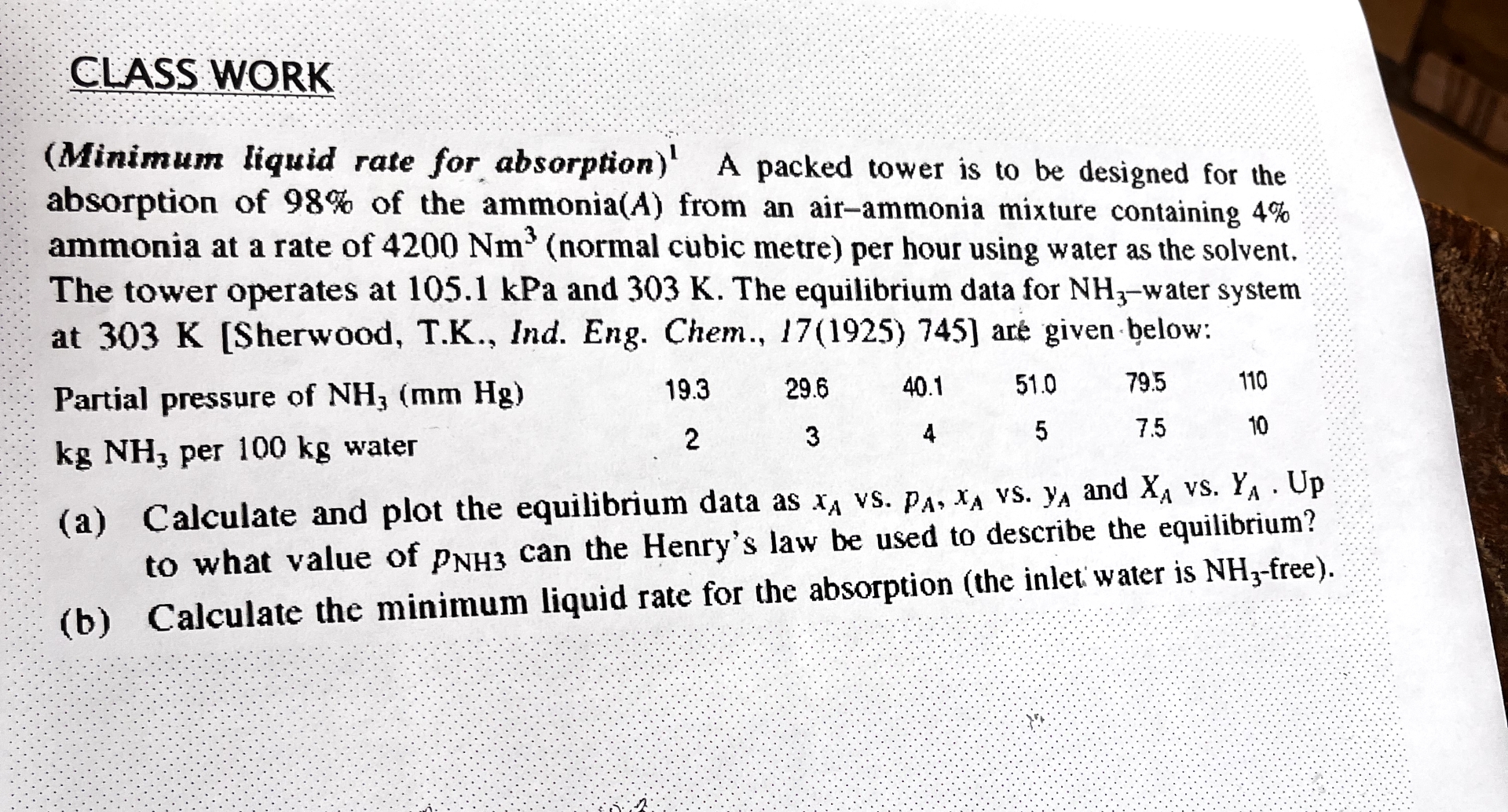
CLASS WORK ( Minimum liquid rate for absorption ) ? 1 A packed tower is to be designed for the absorption of 9 8 %
CLASS WORK
Minimum liquid rate for absorption A packed tower is to be designed for the absorption of of the ammonia from an airammonia mixture containing ammonia at a rate of normal cubic metre per hour using water as the solvent. The tower operates at kPa and The equilibrium data for water system at Sherwood TK Ind. Eng. Chem., are given below:
tablePartial pressure of per water,
a Calculate and plot the equilibrium data as vs vs and vs Up to what value of can the Henry's law be used to describe the equilibrium?
b Calculate the minimum liquid rate for the absorption the inlet water is free
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


