Question
Classify each statement as true or false. Drag the appropriate items to their respective bins. View Available Hint(s) Period 3 elements have six 2p
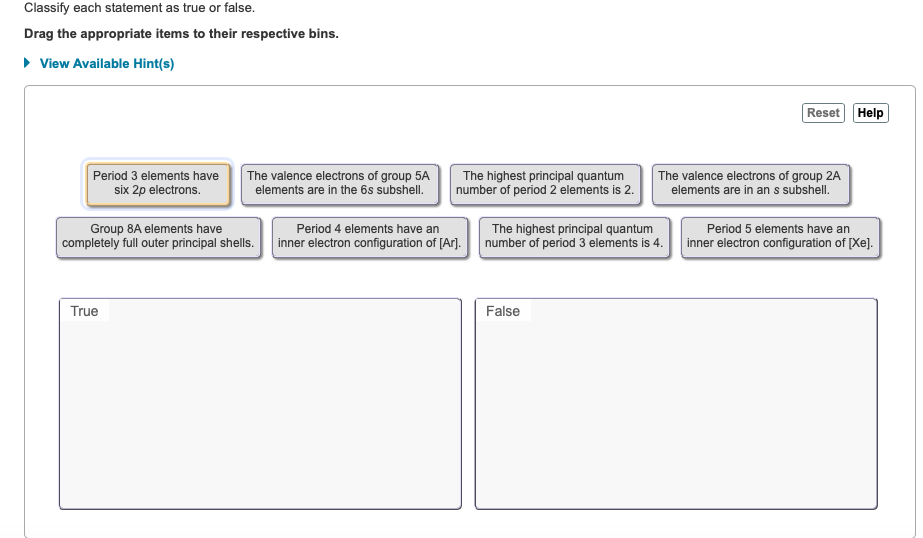
Classify each statement as true or false. Drag the appropriate items to their respective bins. View Available Hint(s) Period 3 elements have six 2p electrons. The valence electrons of group 5A elements are in the 6s subshell. Group 8A elements have completely full outer principal shells. True The highest principal quantum number of period 2 elements is 2. Period 4 elements have an inner electron configuration of [Ar]. The highest principal quantum number of period 3 elements is 4. False Reset Help The valence electrons of group 2A elements are in an s subshell. Period 5 elements have an inner electron configuration of [Xe].
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provide...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Discovering Advanced Algebra An Investigative Approach
Authors: Jerald Murdock, Ellen Kamischke, Eric Kamischke
1st edition
1559539844, 978-1604400069, 1604400064, 978-1559539845
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



