Answered step by step
Verified Expert Solution
Question
1 Approved Answer
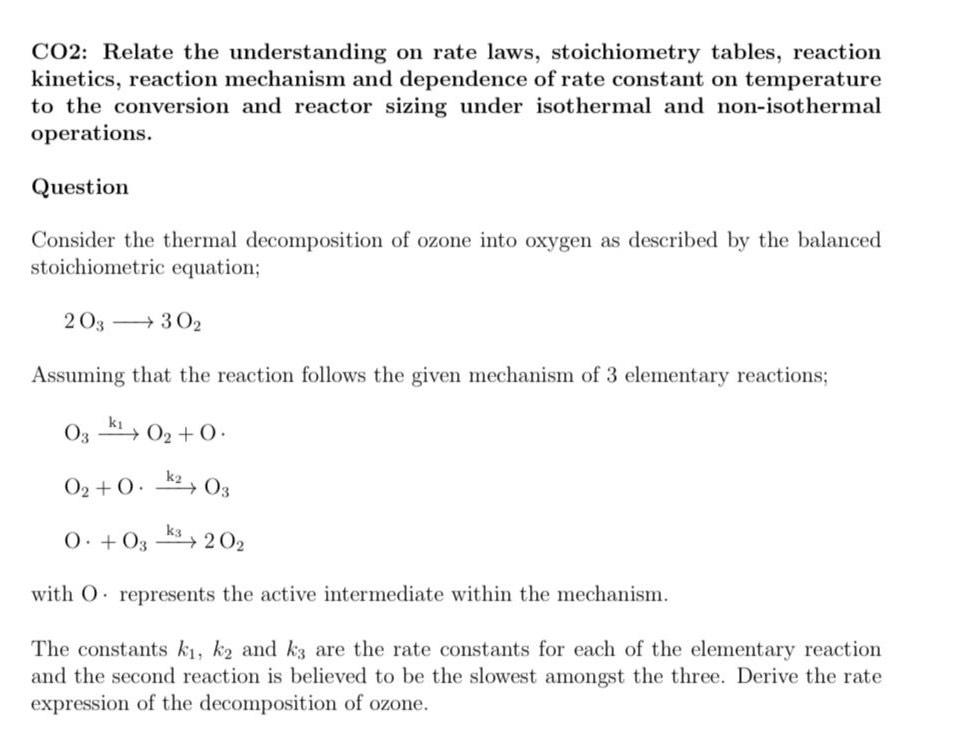
CO 2 : Relate the understanding on rate laws, stoichiometry tables, reaction kinetics, reaction mechanism and dependence of rate constant on temperature to the conversion
CO: Relate the understanding on rate laws, stoichiometry tables, reaction kinetics, reaction mechanism and dependence of rate constant on temperature to the conversion and reactor sizing under isothermal and nonisothermal operations.
Question
Consider the thermal decomposition of ozone into oxygen as described by the balanced stoichiometric equation;
longrightarrow
Assuming that the reaction follows the given mechanism of elementary reactions;
with represents the active intermediate within the mechanism.
The constants and are the rate constants for each of the elementary reaction and the second reaction is believed to be the slowest amongst the three. Derive the rate expression of the decomposition of ozone.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


