Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solid HS is added to 280 ml of a 0.450M CaCl solution until no more CaS is formed (see reaction below). At the end
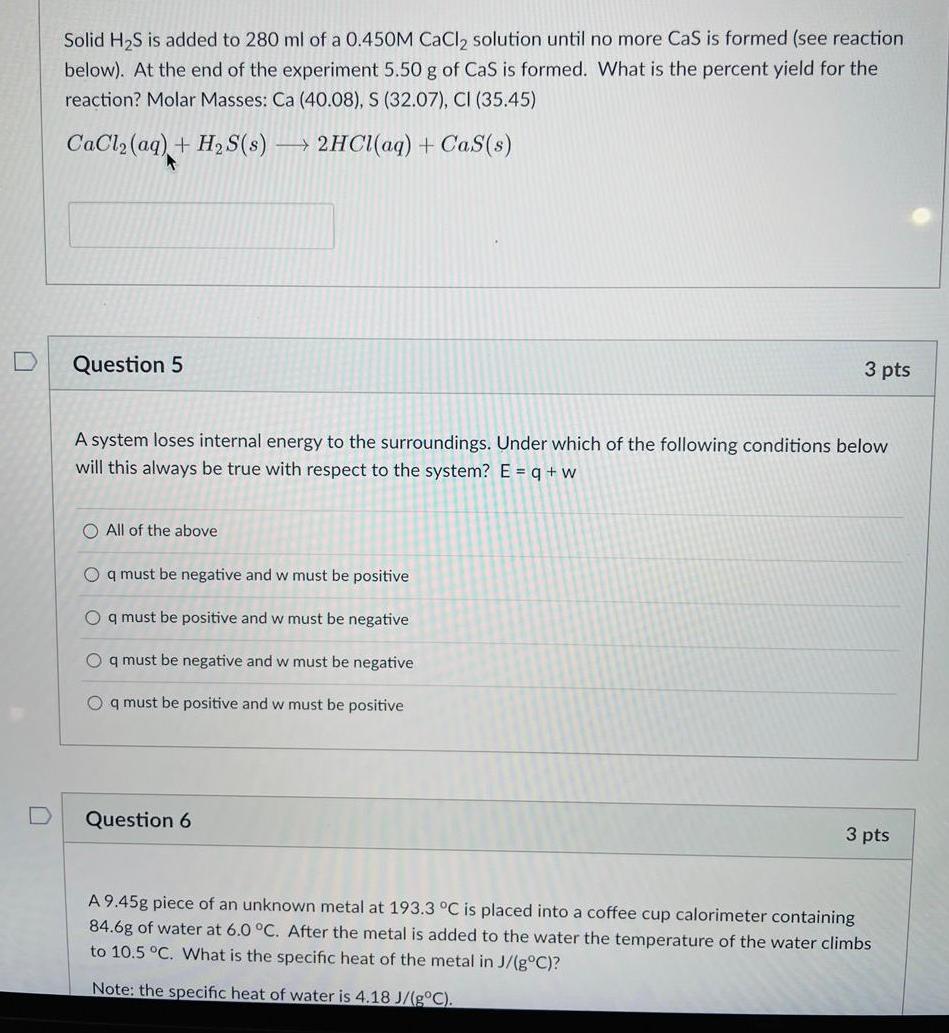
Solid HS is added to 280 ml of a 0.450M CaCl solution until no more CaS is formed (see reaction below). At the end of the experiment 5.50 g of CaS is formed. What is the percent yield for the reaction? Molar Masses: Ca (40.08), S (32.07), CI (35.45) CaCl (aq) + HS(s) 2HCl(aq) + Cas(s) A Question 5 A system loses internal energy to the surroundings. Under which of the following conditions below will this always be true with respect to the system? E = q + w O All of the above Oq must be negative and w must be positive Oq must be positive and w must be negative Oq must be negative and w must be negative Oq must be positive and w must be positive 3 pts Question 6 3 pts A 9.45g piece of an unknown metal at 193.3 C is placed into a coffee cup calorimeter containing 84.6g of water at 6.0 C. After the metal is added to the water the temperature of the water climbs to 10.5 C. What is the specific heat of the metal in J/(gC)? Note: the specific heat of water is 4.18 J/(gC).
Step by Step Solution
★★★★★
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
4 Percent Yield Calculation The balanced chemical equation for the reaction is CaCl2aqH2Ss2HClaqCaSsCaCl2aqH2Ss2HClaqCaSs To calculate the theoretical ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


