Answered step by step
Verified Expert Solution
Question
1 Approved Answer
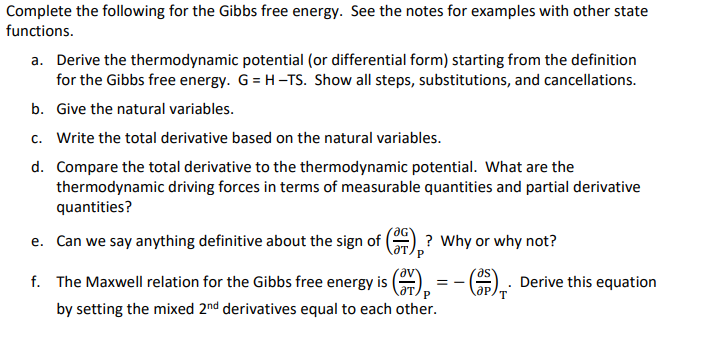
Complete the following for the Gibbs free energy. See the notes for examples with other state functions. a . Derive the thermodynamic potential ( or
Complete the following for the Gibbs free energy. See the notes for examples with other state
functions.
a Derive the thermodynamic potential or differential form starting from the definition
for the Gibbs free energy. G HTS Show all steps, substitutions, and cancellations.
b Give the natural variables.
c Write the total derivative based on the natural variables.
d Compare the total derivative to the thermodynamic potential. What are the
thermodynamic driving forces in terms of measurable quantities and partial derivative
quantities?
e Can we say anything definitive about the sign of Why or why not?
f The Maxwell relation for the Gibbs free energy is Derive this equation
by setting the mixed derivatives equal to each other.Find a vector equation and parametric equations for the line segment that joins P to Q Find a vector equation and parametric equations for the line segment that joins P to Q
P Q
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


