Question
Complete the following partial ICE tables. (a) change (b) 2SO3(9) =2SO2(g) + O2(g) +x change CH4(g) + H2O=CO(g) + 3H2(g) +x
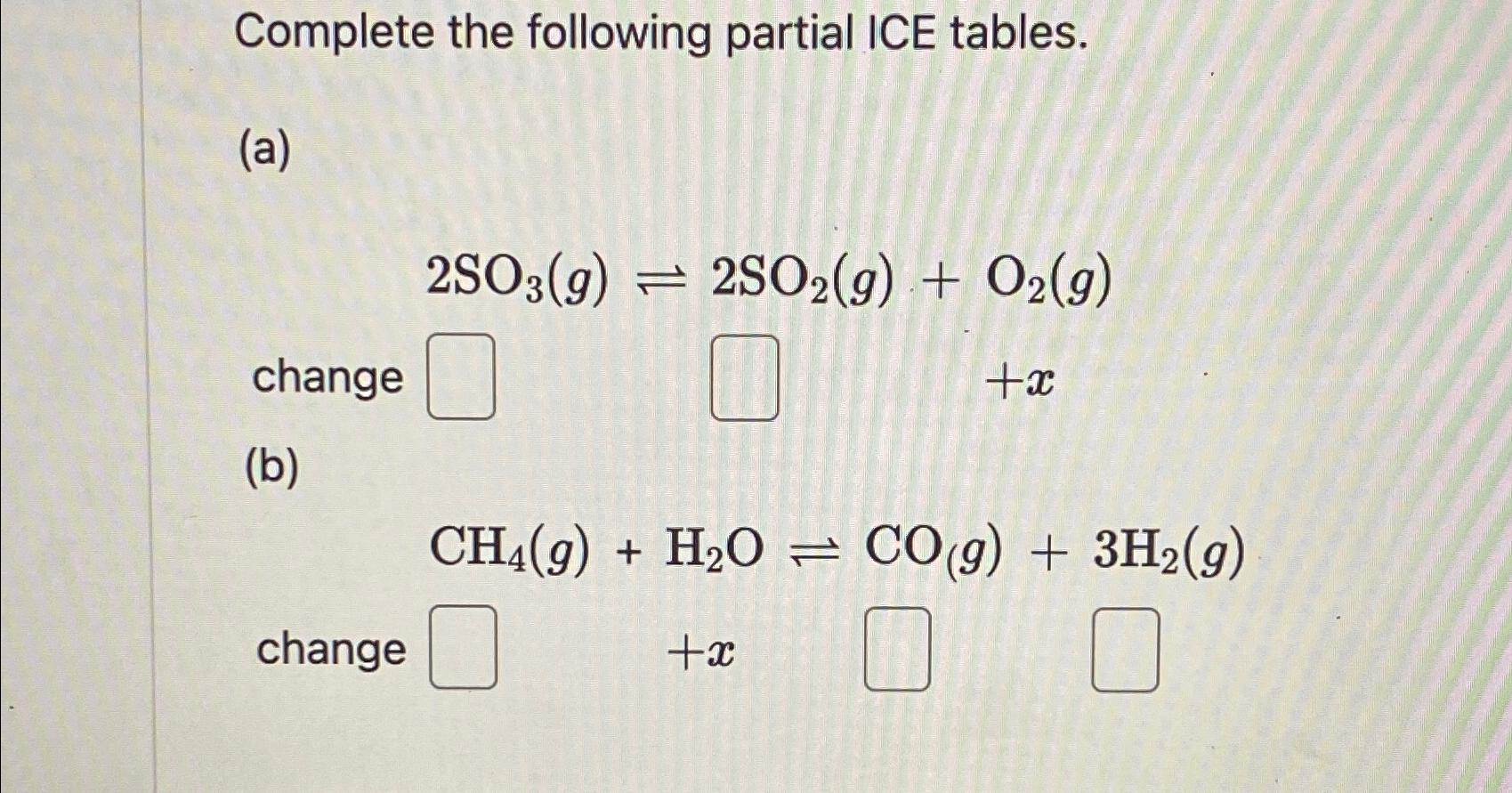
Complete the following partial ICE tables. (a) change (b) 2SO3(9) =2SO2(g) + O2(g) +x change CH4(g) + H2O=CO(g) + 3H2(g) +x
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Completed ICE Tables a 2SO3g 2SO2g O2g Species Initial Change Equilibrium ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Chemical Engineering Thermodynamics
Authors: J. M. Smith, H. C. Van Ness, M. M. Abbott
7th edition
71247084, 978-0071247085
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



