complete the table and show work
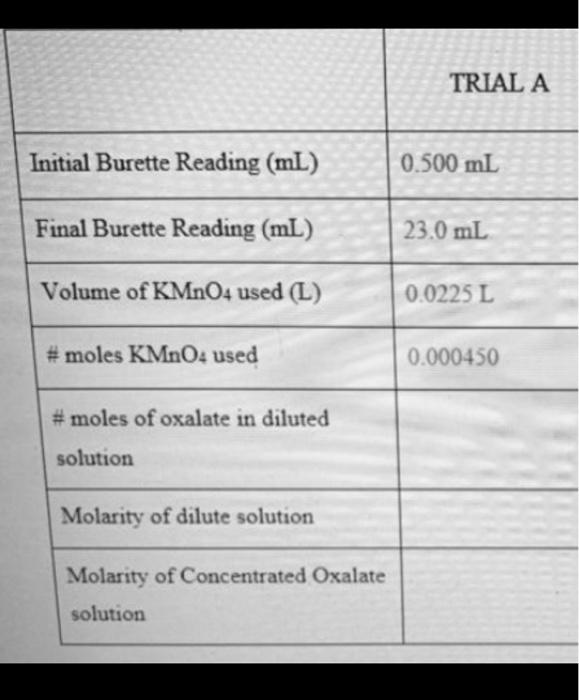
TRIAL A Initial Burette Reading (mL) 0.500 mL Final Burette Reading (mL) 23.0 mL Volume of KMnO4 used (L) 0.0225 L #moles KMnO4 used 0.000450 #moles of oxalate in diluted solution Molarity of dilute solution Molarity of Concentrated Oxalate solution Ihang, (at) + 16+" (as) +o a minta (as) + 8 Hy oce) 590a cas) + 2 mnai (ae) + 16 H Cae) 10Cg (9) + 2 mont'are ) + 8 Hola! Click on the oxalic acid bottle and use the arrow keys on your keyboard to draw up 25.00 mL of solution Click on the pipette and add the oxalic acid to the volumetric flask Repeat the previous two steps to add an additional 25.00 mL of oxalic acid to the volumetric flask Click on the deionized water bottle to fill the volumetric flask to the graduation Click on the volumetric flask and shake it Click on the pipette to draw up 25.00 mL of the dilute oxalic acid solution Click on the pipette to add the solution to the Erlenmeyer Click on the H.SO, bottle and use the arrow keys on your keyboard to adjust the volume in the beaker to 60 ml Click on the beaker to add the sulfuric acid to the Erlenmeyer flask Click on the Erlenmeyer to move it to the hot plate Click on the Erlenmeyer and choose to add the thermometer to the solution Tum on the hot plate by clicking the red button on the left - Heat the sample to a temperature of 60C Click on the Erlenmeyer flask to move it to stir plate under the burette Click on the KMnO bottle to add the potassium permanganate to the burette Click on the burette to record the initial volume Use the arrow keys on your keyboard to add a few drops of KMnOs into the Erlenmeyer flask from the burette. At this point, the colour will change and will require roughly 30 minutes of stirring before it returns clear. DO NOT add additional KMnO until it clears up or you will have to re-start Once the solution goes back to clear, continue the titration until you obtain a permanent purple colour Click on the burette to zoom in and read the final volume. Continue adding KMnO2 until the solution in the Erlenmeyer flask consistently remains a purple colour Titration is complete Repeat this entire procedure until you obtain two titrations within 0.1 mL of each other