Question
Complete the table below for calculating the molar mass of the ionic compound cobalt(III) iodide. Molar mass of ion Mass of ion in one
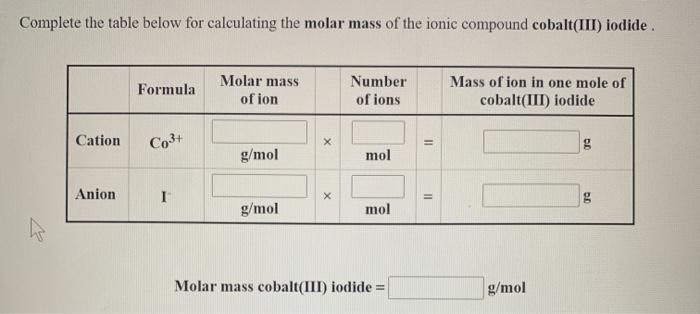
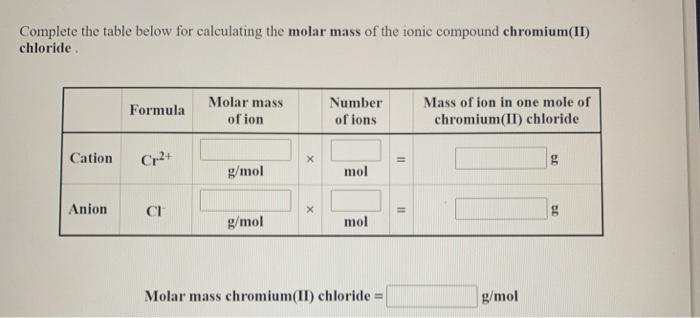
Complete the table below for calculating the molar mass of the ionic compound cobalt(III) iodide. Molar mass of ion Mass of ion in one mole of Formula Number of ions cobalt(III) iodide Cation Co3+ g g/mol mol Anion I g g/mol mol Molar mass cobalt(III) iodide = 2 X 11 II g/mol Complete the table below for calculating the molar mass of the ionic compound chromium(II) chloride. Formula Molar mass of ion Number of ions Mass of ion in one mole of chromium(II) chloride Cation Cr2+ X g g/mol mol Anion CH X g g/mol mol Molar mass chromium(II) chloride = 11 11 g/mol
Step by Step Solution
3.52 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Solution 1 Molar mass of Tonic Compound Cobal klin ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Horngrens Financial and Managerial Accounting
Authors: Tracie L. Nobles, Brenda L. Mattison, Ella Mae Matsumura
5th edition
9780133851281, 013385129x, 9780134077321, 133866297, 133851281, 9780133851298, 134077326, 978-0133866292
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



