Question
Complete the table below with the correct molecularity and rate law for each elementary reaction. Unimolecular rate= K[AP Elementary Step A products 2A ->
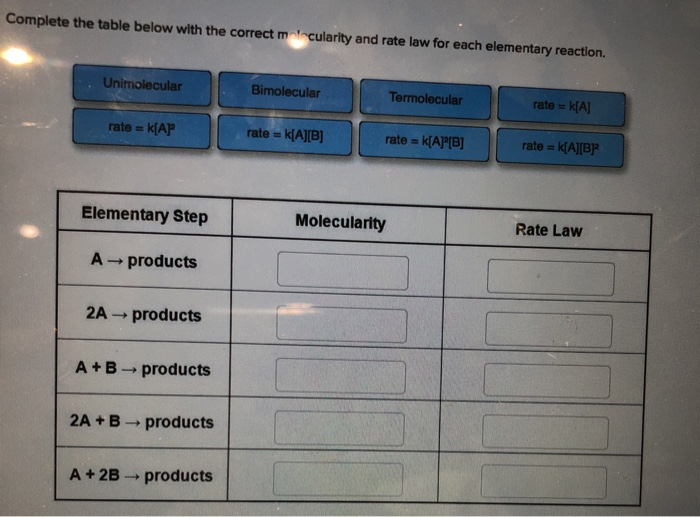
Complete the table below with the correct molecularity and rate law for each elementary reaction. Unimolecular rate= K[AP Elementary Step A products 2A -> products A+B products 2A + B products -> A + 2B - products Bimolecular rate == k[A][B] Termolecular rate == Molecularity IN k[A][B] rate = k[A] rate == k[A][B] Rate Law
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics Principles And Methods
Authors: Richard A. Johnson, Gouri K. Bhattacharyya
8th Edition
1119497116, 978-1119497110
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



